Genetic evidence that Legionella pneumophila RpoS modulates expression of the transmission phenotype in both the exponential phase and the stationary phase - PubMed (original) (raw)
Genetic evidence that Legionella pneumophila RpoS modulates expression of the transmission phenotype in both the exponential phase and the stationary phase
Michael A Bachman et al. Infect Immun. 2004 May.
Abstract
The opportunistic pathogen Legionella pneumophila alternates between two states: replication within phagocytes and transmission between host amoebae or macrophages. In broth cultures that model this life cycle, during the replication period, CsrA inhibits expression of transmission traits. When nutrients become limiting, the alarmone (p)ppGpp accumulates and the sigma factors RpoS and FliA and the positive activators LetA/S and LetE promote differentiation to the transmissible form. Here we show that when cells enter the postexponential growth phase, RpoS increases expression of the transmission genes fliA, flaA, and mip, factors L. pneumophila needs to establish a new replication niche. In contrast, in exponential (E)-phase cells whose (p)ppGpp levels are low, rpoS inhibits expression of transmission traits, on the basis of three separate observations. First, rpoS RNA levels peak in the E phase, suggestive of a role for RpoS during replication. Second, in multiple copies, rpoS decreases the amounts of csrA, letE, fliA, and flaA transcripts and inhibits the transmission traits of motility, infectivity, and cytotoxicity. Third, rpoS blocks expression of cytotoxicity and motility by E-phase bacteria that have been induced to express the LetA activator ectopically. The data are discussed in the context of a model in which the alarmone (p)ppGpp enables RpoS to outcompete other sigma factors for binding to RNA polymerase to promote transcription of transmission genes, while LetA/S acts in parallel to relieve CsrA posttranscriptional repression of the transmission regulon. By coupling transcriptional and posttranscriptional control pathways, intracellular L. pneumophila could respond to stress by rapidly differentiating to a transmissible form.
Figures
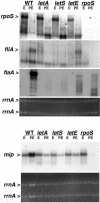
FIG. 1.
Accumulation of rpoS, fliA, flaA, and mip mRNAs by E- and PE-phase wild-type (WT) and mutant L. pneumophila. Northern analysis was performed on 10 μg of total RNA collected from E-phase (OD600, 0.8 to 1.2) and PE-phase (OD600, 3.1 to 3.6) cultures of wild-type Lp02 bacteria and _letA_-22, _letS_-36, _letE_-121, and rpoS mutant bacteria with a biotin-labeled ssDNA probe. Hybridization was detected with streptavidin conjugated to alkaline phosphatase and visualized by CDP-Star (Tropix). The hybridization pattern shown is representative of results obtained from at least two independent sets of RNA samples. The relative amount of total RNA in each sample is demonstrated by ethidium bromide staining of rrnA from the formaldehyde gel prior to membrane transfer. Greater hybridization to mip in the wild-type PE-phase sample is due to a higher concentration of total RNA, as demonstrated by ethidium bromide staining of rrnA.
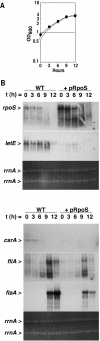
FIG. 2.
RpoS overexpression represses letE, csrA, fliA, and flaA expression by a mechanism that does not affect L. pneumophila growthin broth. (A) OD600 of cultures of wild-type Lp02 containing the vector pMB384 (open circles) or pLrpoS (pMB391, closed circles). At each time point shown, aliquots were removed, centrifuged, permeabilized with Trizol reagent, extracted for total RNA as directed by the manufacturer (Invitrogen), and analyzed as described for panel B. (B) Northern analysis was performed on 10 μg of total RNA collected as described for panel A. The same membrane was stripped and reprobed for each transcript. The relative amount of total RNA in each sample is demonstrated by ethidium bromide staining of rrnA from the formaldehyde gel prior to membrane transfer. The results shown are representative of two sets of RNA samples collected from independent cultures at similar optical densities. WT, wild type.
FIG. 3.
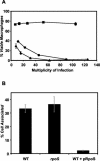
Multicopy expression of rpoS eliminates cytotoxicity and infectivity. (A) Contact-dependent cytotoxicity for macrophages. Macrophages were incubated for 1 h with samples of PE-phase wild-type Lp02 containing the vector pMB384 (circles), pLrpoS harboring a 6.8-kb rpoS locus (pMB391, squares), or pLrpoS::kan carrying an rpoS::kan mutation in the same locus (pMB392, triangles). Macrophage viability was assessed by quantifying reduction of the colorimetric dye Alamar blue. Shown are means and standard errors of triplicate samples from one representative experiment of three performed. (B) Efficiency of macrophage infection. Macrophages were infected at a multiplicity of infection of 0.2 with the following PE-phase strains: wild-type (WT) strain Lp02 containing the vector pMB384, rpoS mutant strain MB379 (rpoS::kan) containing pMB384 and the wild type plus pLrpoS. The percentage of each L. pneumophila inoculum that was cell associated after a 2-h infection with 2.5 × 105 macrophages is shown. The results shown are means of macrophage samples infected in triplicate; error bars represent standard deviations.
FIG. 4.
RpoS represses LetA-induced cytotoxicity in the E phase. After overnight growth in the presence (closed symbols) or absence (open symbols) of 200 μM IPTG to induce expression from pLetA, samples of E-phase wild-type Lp02 (circles) or rpoS mutant MB379 (squares) were incubated for 1 h with macrophages. Macrophage viability was assessed by quantifying reduction of the colorimetric dye Alamar blue. Shown are means and standard errors of triplicate samples from one representative experiment of six performed.
Similar articles
- The LetE protein enhances expression of multiple LetA/LetS-dependent transmission traits by Legionella pneumophila.
Bachman MA, Swanson MS. Bachman MA, et al. Infect Immun. 2004 Jun;72(6):3284-93. doi: 10.1128/IAI.72.6.3284-3293.2004. Infect Immun. 2004. PMID: 15155631 Free PMC article. - A two-component regulator induces the transmission phenotype of stationary-phase Legionella pneumophila.
Hammer BK, Tateda ES, Swanson MS. Hammer BK, et al. Mol Microbiol. 2002 Apr;44(1):107-18. doi: 10.1046/j.1365-2958.2002.02884.x. Mol Microbiol. 2002. PMID: 11967072 - RpoS co-operates with other factors to induce Legionella pneumophila virulence in the stationary phase.
Bachman MA, Swanson MS. Bachman MA, et al. Mol Microbiol. 2001 Jun;40(5):1201-14. doi: 10.1046/j.1365-2958.2001.02465.x. Mol Microbiol. 2001. PMID: 11401723 - Differentiate to thrive: lessons from the Legionella pneumophila life cycle.
Molofsky AB, Swanson MS. Molofsky AB, et al. Mol Microbiol. 2004 Jul;53(1):29-40. doi: 10.1111/j.1365-2958.2004.04129.x. Mol Microbiol. 2004. PMID: 15225301 Review. - The small regulatory RNA Lpr10 regulates the expression of RpoS in Legionella pneumophila.
Saoud J, Carrier MC, Massé É, Faucher SP. Saoud J, et al. Mol Microbiol. 2021 Apr;115(4):789-806. doi: 10.1111/mmi.14644. Epub 2020 Dec 10. Mol Microbiol. 2021. PMID: 33191583 Review.
Cited by
- The many forms of a pleomorphic bacterial pathogen-the developmental network of Legionella pneumophila.
Robertson P, Abdelhady H, Garduño RA. Robertson P, et al. Front Microbiol. 2014 Dec 22;5:670. doi: 10.3389/fmicb.2014.00670. eCollection 2014. Front Microbiol. 2014. PMID: 25566200 Free PMC article. Review. - The Legionella pneumophila LetA/LetS two-component system exhibits rheostat-like behavior.
Edwards RL, Jules M, Sahr T, Buchrieser C, Swanson MS. Edwards RL, et al. Infect Immun. 2010 Jun;78(6):2571-83. doi: 10.1128/IAI.01107-09. Epub 2010 Mar 29. Infect Immun. 2010. PMID: 20351136 Free PMC article. - Knowledge to Predict Pathogens: Legionella pneumophila Lifecycle Systematic Review Part II Growth within and Egress from a Host Cell.
Mraz AL, Weir MH. Mraz AL, et al. Microorganisms. 2022 Jan 11;10(1):141. doi: 10.3390/microorganisms10010141. Microorganisms. 2022. PMID: 35056590 Free PMC article. Review. - The phtC-phtD locus equips Legionella pneumophila for thymidine salvage and replication in macrophages.
Fonseca MV, Sauer JD, Crepin S, Byrne B, Swanson MS. Fonseca MV, et al. Infect Immun. 2014 Feb;82(2):720-30. doi: 10.1128/IAI.01043-13. Epub 2013 Dec 2. Infect Immun. 2014. PMID: 24478086 Free PMC article. - The Hfq homolog in Legionella pneumophila demonstrates regulation by LetA and RpoS and interacts with the global regulator CsrA.
McNealy TL, Forsbach-Birk V, Shi C, Marre R. McNealy TL, et al. J Bacteriol. 2005 Feb;187(4):1527-32. doi: 10.1128/JB.187.4.1527-1532.2005. J Bacteriol. 2005. PMID: 15687220 Free PMC article.
References
- Ahmer, B. M., J. van Reeuwijk, P. R. Watson, T. S. Wallis, and F. Heffron. 1999. Salmonella SirA is a global regulator of genes mediating enteropathogenesis. Mol. Microbiol. 31:971-982. - PubMed
- Bachman, M. A., and M. S. Swanson. 2001. RpoS co-operates with other factors to induce Legionella pneumophila virulence in the stationary phase. Mol. Microbiol. 40:1201-1214. - PubMed
- Berger, K. H., and R. R. Isberg. 1993. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol. Microbiol. 7:7-19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources