Cdc42 and the actin-related protein/neural Wiskott-Aldrich syndrome protein network mediate cellular invasion by Cryptosporidium parvum - PubMed (original) (raw)
Cdc42 and the actin-related protein/neural Wiskott-Aldrich syndrome protein network mediate cellular invasion by Cryptosporidium parvum
Xian-Ming Chen et al. Infect Immun. 2004 May.
Abstract
Cryptosporidium parvum invasion of epithelial cells involves host cell membrane alterations which require a remodeling of the host cell actin cytoskeleton. In addition, an actin plaque, possibly associated with the dense-band region, forms within the host cytoplasm at the host-parasite interface. Here we show that Cdc42 and RhoA, but not Rac1, members of the Rho family of GTPases, are recruited to the host-parasite interface in an in vitro model of human biliary cryptosporidiosis. Interestingly, activation of Cdc42, but not RhoA, was detected in the infected cells. Neural Wiskott-Aldrich syndrome protein (N-WASP) and p34-Arc, actin-regulating downstream effectors of Cdc42, were also recruited to the host-parasite interface. Whereas cellular expression of a constitutively active mutant of Cdc42 promoted C. parvum invasion, overexpression of a dominant negative mutant of Cdc42, or depletion of Cdc42 mRNA by short interfering RNA-mediated gene silencing, inhibited C. parvum invasion. Expression of the WA fragment of N-WASP to block associated actin polymerization also inhibited C. parvum invasion. Moreover, inhibition of host cell Cdc42 activation by dominant negative mutation inhibited C. parvum-associated actin remodeling, membrane protrusion, and dense-band formation. In contrast, treatment of cells with a Rho inhibitor, exoenzyme C3, or cellular overexpression of dominant negative mutants of RhoA and Rac1 had no effect on C. parvum invasion. These data suggest that C. parvum invasion of target epithelia results from the organism's ability to activate a host cell Cdc42 GTPase signaling pathway to induce host cell actin remodeling at the attachment site.
Figures
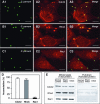
FIG. 1.
C. parvum recruits host cell Cdc42 and RhoA to the host-parasite interface but activates only Cdc42 in infected biliary epithelial cells. Biliary epithelial cells were exposed to C. parvum sporozoites and recruitment and activation of Rho GTP-binding proteins were then determined by confocal microscopy and pull-down approach, respectively. Accumulation of host cell Cdc42 (A1 to A3) and RhoA (B1 to B3) was found at the parasite-epithelial cell interface in infected biliary epithelial cells after exposure to C. parvum for 2 h. (C1 to C3) Rac1, another small GTP-binding protein, did not show accumulation at the host cell-parasite interface. Labels indicate staining of C. parvum or proteins or the mergers of the corresponding red and green panels. (D) Quantitative analysis of accumulation of Cdc42, RhoA and Rac1 at the host-parasite interface. (E) GST pull-down assay of Rho GTP-binding protein activation in biliary epithelial cells after exposure to C. parvum sporozoites for 1 h. The whole lysates showed similar bands to Cdc42, RhoA, or Rac1, suggesting no change at the total protein level in infected cells after C. parvum infection. GST pull-down using GST-CRIB (which specifically binds to the GTP-bound form of Cdc42) showed a much stronger band in C. parvum infected cells than that in the normal control or sham-infected cells, suggesting activation of Cdc42 in biliary epithelial cells after C. parvum infection. No significant increase of the band for RhoA or Rac1 was found in the pull-down assay using GST-RBD (which binds to GTP-RhoA) or GST-PBD (which binds to GTP-Rac1), respectively. Error bars, standard errors of the means; scale bars = 5 μm.
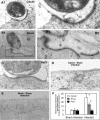
FIG. 2.
Accumulation of Cdc42 and RhoA at the host-parasite interface as revealed by immunoelectron microscopy. Biliary epithelial cells were exposed to C. parvum sporozoites for 2 h followed by immunoelectron microscopy. (A1 and B1) Immunogold labeling of Cdc42 (A1) and RhoA (B1) at the host-parasite interface in infected cells. (A2 and B2) Boxed regions of A1 and A2, respectively, shows at a higher magnification. (C) Immunogold labeling of Rac1 at the host-parasite interface. (E and F) Representative images of gold particle labeling of Cdc42 and RhoA in sham-infected control cells. (G) Quantitative analysis of immunogold particles for Cdc42, RhoA and Rac1 around the dense-band area. Immunogold particles at randomly selected apical membrane areas in sham-infected cells were used as the controls. *, P < 0.05 (compared with sham-infected controls); error bars, standard errors of the means; scale bars = 0.1 μm.
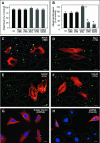
FIG. 3.
C. parvum invasion of biliary epithelial cells requires the activation of host cell Cdc42, but not RhoA and Rac1. Cells were transfected with either a consitutively active mutant of Ccd42 or dominant negative mutants of Cdc42, RhoA, or Rac1 and then exposed to C. parvum followed by immunofluorescent microscopy. Some cells were transfected with a vector encoding an shRNA toward Cdc42 before exposure to C. parvum. (A) Attachment assay in prefixed cells after a 2-h exposure to C. parvum sporozoites shows no significant difference of C. parvum attachment in all the treated cells. (B) Attachment-invasion assay in nonfixed cells after a 2-h exposure to C. parvum sporozoites. A significant increase of infection rate was found in cells transfected with Cdc42 (Q61L) and a significant decrease of infection rate was detected in cells transfected withCdc42 (17N) or Cdc42 siRNA, but not RhoA (19N) and Rac1(17N) or cells treated with exoenzyme C3. (C to H) Representative confocal images of cells of various treatment exposed to C. parvum for 2 h. Transfected cells were identified by immunostaining using an antibody to the C-Myc epitope tag. No significant different of C. parvum infection was found between nontransfected cells as outlined (C and D) and cells transfected with RhoA (19N) (stained in red in C) or Rac1 (19N) (in red in D). More C. parvum parasites were detected in cells transfected with Cdc42 (61L) (in red in E) and much fewer C. parvum parasites were found in cells transfected with Cdc42 (17N) (in red in F) compared with nontransfected cells (as outlined in E and F). Whereas cells transfected with the empty shRNA vector displayed a normal Cdc42 cellular expression and a similar infection pattern as nontransfected cells (G), cells transfected with shRNA to Cdc42 showed no obvious expression of Cdc42 (with nucleus staining with DAPI but absence of Cdc42 expression in H) and a marked decrease of C. parvum infection compared with nontransfected cells (shown expression of Cdc42 in H). *, P < 0.05 (compared with no-inhibitor treated or nontransfected controls); error bars, standard errors of the means; scale bars = 5 μm.
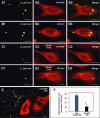
FIG. 4.
Downstream effectors of Ccd42 and C. parvum invasion of biliary epithelial cells. (A and B) C. parvum stimulates the accumulation of host cell N-WASP and p34-Arc at the host-parasite interface in infected biliary epithelial cells. Biliary epithelial cells were exposed to C. parvum sporozoites for 2 h and then costained of C. parvum with associated proteins followed by confocal microscopy. (C and D) Inhibition of Cdc42 blocks _C. parvum_-induced N-WASP and p34-Arc accumulation at the host-parasite interface. Biliary epithelial cells were transfected with Cdc42(17N), selected by antibiotic section and then exposed to C. parvum sporozoites for 2 h followed by confocal microscopy. No obvious accumulation of N-WASP (C1 to C3) and p34-Arc (D1 to D3) was detected at the host-parasite interface in the transfected cells. (E) Representative confocal image of cells which were transfected with N-WASP-WA and then exposed to C. parvum for 2 h. Much fewer C. parvum parasites were found in cells transfected with N-WASP-WA (stained in red using an antibody to the C-Myc epitope tag in F) compared with nontransfected cells (as outlined in E). (F) Quantitative analysis of attachment-invasion of C. parvum in nonfixed cells transfected with N-WASP-WA after a 2-h exposure to C. parvum sporozoites. A significant decrease of infection rate was detected in cells transfected with N-WASP-WA. *, P < 0.05 (compared with nontransfected controls); error bars, standard errors of the means; scale bars = 5 μm.
FIG. 5.
Inhibition of host cell Cdc42 activation hampers _C. parvum_-induced actin reorganization, host cell membrane protrusion and dense-band formation at the host-parasite interface. Cdc42 (17N)-transfected cells were selected by antibiotic selection and then exposed to C. parvum for 2 h followed by confocal microscopy or SEM or TEM. (A and B) Confocal microscopy shows that C. parvum induces host cell actin accumulation at the host-parasite interface in empty vector-transfected cells (A1 to A3), but not in Cdc42 (17N)-transfected cells (B1 to B3). (C and D) Electron micrographs show C. parvum attachment and invasion of empty vector control cells, with host cell membrane protrusion and microvilli around the organism (C) and the dense-band underlying the parasitophorous vacuole (arrows in D). (E and F) C. parvum can only attach to the membrane surface of selected Cdc42 (17N) transfected cells. No obvious host cell membrane protrusion and dense-band formation at the host-parasite interface (F). Scale bar = 0.5 μm.
Similar articles
- Phosphatidylinositol 3-kinase and frabin mediate Cryptosporidium parvum cellular invasion via activation of Cdc42.
Chen XM, Splinter PL, Tietz PS, Huang BQ, Billadeau DD, LaRusso NF. Chen XM, et al. J Biol Chem. 2004 Jul 23;279(30):31671-8. doi: 10.1074/jbc.M401592200. Epub 2004 May 7. J Biol Chem. 2004. PMID: 15133042 - Cryptosporidium parvum invasion of biliary epithelia requires host cell tyrosine phosphorylation of cortactin via c-Src.
Chen XM, Huang BQ, Splinter PL, Cao H, Zhu G, McNiven MA, LaRusso NF. Chen XM, et al. Gastroenterology. 2003 Jul;125(1):216-28. doi: 10.1016/s0016-5085(03)00662-0. Gastroenterology. 2003. PMID: 12851885 - Cryptosporidium parvum infection requires host cell actin polymerization.
Elliott DA, Coleman DJ, Lane MA, May RC, Machesky LM, Clark DP. Elliott DA, et al. Infect Immun. 2001 Sep;69(9):5940-2. doi: 10.1128/IAI.69.9.5940-5942.2001. Infect Immun. 2001. PMID: 11500478 Free PMC article. - Host cell actin remodeling in response to Cryptosporidium.
O'Hara SP, Small AJ, Chen XM, LaRusso NF. O'Hara SP, et al. Subcell Biochem. 2008;47:92-100. doi: 10.1007/978-0-387-78267-6_7. Subcell Biochem. 2008. PMID: 18512344 Review. - Regulation of cancer cell motility through actin reorganization.
Yamazaki D, Kurisu S, Takenawa T. Yamazaki D, et al. Cancer Sci. 2005 Jul;96(7):379-86. doi: 10.1111/j.1349-7006.2005.00062.x. Cancer Sci. 2005. PMID: 16053508 Free PMC article. Review.
Cited by
- Survival of protozoan intracellular parasites in host cells.
Leirião P, Rodrigues CD, Albuquerque SS, Mota MM. Leirião P, et al. EMBO Rep. 2004 Dec;5(12):1142-7. doi: 10.1038/sj.embor.7400299. EMBO Rep. 2004. PMID: 15577928 Free PMC article. Review. - Cryptosporidium parvum Elongation Factor 1α Participates in the Formation of Base Structure at the Infection Site During Invasion.
Yu X, Guo F, Mouneimne RB, Zhu G. Yu X, et al. J Infect Dis. 2020 May 11;221(11):1816-1825. doi: 10.1093/infdis/jiz684. J Infect Dis. 2020. PMID: 31872225 Free PMC article. - Role of Host Small GTPases in Apicomplexan Parasite Infection.
Paone S, Olivieri A. Paone S, et al. Microorganisms. 2022 Jul 7;10(7):1370. doi: 10.3390/microorganisms10071370. Microorganisms. 2022. PMID: 35889089 Free PMC article. Review. - Subverting Host Cell P21-Activated Kinase: A Case of Convergent Evolution across Pathogens.
John Von Freyend S, Kwok-Schuelein T, Netter HJ, Haqshenas G, Semblat JP, Doerig C. John Von Freyend S, et al. Pathogens. 2017 Apr 21;6(2):17. doi: 10.3390/pathogens6020017. Pathogens. 2017. PMID: 28430160 Free PMC article. Review. - The role of atypical MAP kinase 4 in the host interaction with Cryptosporidium parvum.
Watanabe N, Bando H, Murakoshi F, Sakurai R, Kabir MHB, Fukuda Y, Kato K. Watanabe N, et al. Sci Rep. 2023 Jan 19;13(1):1096. doi: 10.1038/s41598-023-28269-w. Sci Rep. 2023. PMID: 36658270 Free PMC article.
References
- Aji, T., T. Flanigan, R. Marshall, C. Kaetzel, and M. Aikawa. 1991. Ultrastructural study of asexual development of Cryptosporidium parvum in a human intestinal cell line. J. Protozool. 38:82S-84S. - PubMed
- Benard, V., B. P. Bohl, and G. M. Bokoch. 1999. Characterization of Rac and Cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J. Biol. Chem. 274:13198-13204. - PubMed
- Bendayan, M. 1984. Concentration of amylase along its secretory pathway in the pancreatic acinar cell as revealed by high resolution immunocytochemistry. Histochem. J. 16:85-108. - PubMed
- Bonnin, A., A. Lapillonne, T. Petrella, J. Lopez, C. Chaponnier, G. Gabbiani, S. Robine, and J. F. Dubremetz. 1999. Immunodetection of the microvillous cytoskeleton molecules villin and ezrin in the parasitophorous vacuole wall of Cryptosporidium parvum (Protozoa: Apicomplexa). Eur. J. Cell Biol. 78:794-801. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK044650/DK/NIDDK NIH HHS/United States
- DK-24031/DK/NIDDK NIH HHS/United States
- R01 DK057993/DK/NIDDK NIH HHS/United States
- DK-44650/DK/NIDDK NIH HHS/United States
- R56 DK044650/DK/NIDDK NIH HHS/United States
- DK-57993/DK/NIDDK NIH HHS/United States
- R01 DK024031/DK/NIDDK NIH HHS/United States
- R37 DK044650/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous