Type IV pilus retraction in pathogenic Neisseria is regulated by the PilC proteins - PubMed (original) (raw)
Comparative Study
. 2004 May 5;23(9):2009-17.
doi: 10.1038/sj.emboj.7600200. Epub 2004 Apr 22.
Affiliations
- PMID: 15103324
- PMCID: PMC404320
- DOI: 10.1038/sj.emboj.7600200
Comparative Study
Type IV pilus retraction in pathogenic Neisseria is regulated by the PilC proteins
Philippe C Morand et al. EMBO J. 2004.
Abstract
Pathogenic Neisseria express type IV pili (tfp), which have been shown to play a central role in the interactions of bacteria with their environment. The regulation of piliation thus constitutes a central element in bacterial life cycle. The PilC proteins are outer membrane-associated proteins that have a key role in tfp biogenesis since PilC-null mutants appear defective for fibre expression. Moreover, tfp are also subjected to retraction, which is under the control of the PilT nucleotide-binding protein. In this work, we bring evidence that fibre retraction involves the translocation of pilin subunits to the cytoplasmic membrane. Furthermore, by engineering meningococcal strains that harbour inducible pilC genes, and with the use of meningococcus-cell interaction as a model for the sequential observation of fibre expression and retraction, we show that the PilC proteins regulate PilT-mediated fibre retraction.
Figures
Figure 1
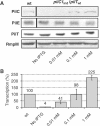
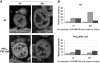
Phenotypes associated with the induction of pilC1. Strains NmC2SC1WEa (PilC1ind/PilTwt) and Nm2C4.3 (wild type), as reference, were grown on GC-agar plates supplemented with IPTG (concentration up to 1 mM). (A) Western blot analysis. Total bacterial extracts were analysed for the presence of PilC, pilin (PilE), PilT and RmpM (extracts calibration). Semiquantitative analysis of Western blots showed an approximate two-fold overexpression of PilC1ind with 1 mM IPTG, compared with PilC1wt. (B) Comparison of the transcriptional level of _pilC1_ind (NmC2SC1WEa) for IPTG concentration up to 1 mM, with that of _pilC1_wt (Nm2C4.3, 100% reference). The transcription of _pilC1_ind is expressed as a percentage of that of _pilC1_wt. Data are mean±standard error of the mean, resulting from at least six measurements.
Figure 2
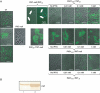
Piliation phenotypes in relation with the balance between PilC1 and PilT. (A) Strains Nm2C4.3 (wild type), PilE-null, PilC-null/PilTwt, PilCwt/PilT-null, PilC1ind/PilTwt (NmC2SC1WEa), PilC1ind/PilT-null (NmC1ITSE) and PilC1ind/PilT∷E (NmC1IESK) were grown on GC-agar plates supplemented with up to 1 mM IPTG. Piliation was assessed by immunofluorescence staining of pilin. Bacteria appear in phase contrast and pilin in green; the arrows show pili. Unless stated, the scale bar is 10 μm. In a PilT+ background, piliation correlates with the expression of PilC1 (panel PilC1ind/PilTwt), whereas abolition of PilT-driven retraction leads to hyperpiliation, independent of the amount of PilC1 (panel PilC1ind/PilT-null). For each level of pilC1 induction, increased expression of PilT leads to decreased piliation (panels PilC1ind/PilTwt and PilC1ind/PilT∷E). (B) Immunolabelling of PilT: Similar amounts of total protein extracts of wild-type (Nm2C4.3, lane 1) and PilC1ind/PilT∷E (NmC1IESK, lane 2) strains were used for Western blotting. Expression of the pilT_∷_E construct leads to increased levels of PilT, compared to the wild type.
Figure 3
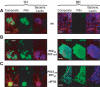
Piliation status of meningococci during adhesion. The wild-type (Nm2C4.3, panel A), PilCwt/PilT-null (panel B) and PilC1ind/PilTwt (NmC2SC1WEa, panel C) strains were allowed to adhere onto HUVECs for 1 h (localised adhesion) or 8 h (intimate adhesion). Bacteria appear blue, f-actin red and pili green. The scale bar is 10 μm. (A) Adhesion of the wild type is characterised by the loss of pili at intimate adhesion. (B) Tfp retraction relies on the presence of PilT: The PilT-null strain remains piliated at a time corresponding to intimate adhesion for the wild type. (C) Constant induction of pilC1 with IPTG in the PilC1ind/PilTwt strain prevents fibre retraction at late adhesion times.
Figure 4
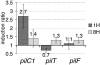
Transcriptional measurements for pilC1, pilT and pilF during adhesion. Relative transcription levels of pilC1, pilT and pilF were measured at localised (1H) and intimate (8H) adhesion stages. The induction ratio compares the transcription of genes in cell-associated bacteria with that of meningococci grown in the absence of human cells (infection medium alone). The transcription of pilC2 was not monitored since it was previously shown that it is not affected by adhesion (Taha et al, 1998). Data represent mean±standard error of the mean, resulting from at least six independent measurements.
Figure 5
Pilin gold labelling on ultra-thin sections of adhering meningococci. Wild-type (Nm2C4.3) or PilCwt/PilT-null strains were allowed to adhere to HUVECs for 1 h (1H) to 8 h (8H). (A) In the wild type (upper panel), no gold beads are seen in the external milieu or associated with the outer membrane at intimate adhesion (8H), whereas numerous beads are observed on the surface of the bacterial cell or along pili at early time point (1H). In the absence of PilT-mediated retraction (lower panel), similar pilin distribution patterns are observed for the PilCwt/PilT-null strain throughout adhesion (1H and 8H). (B) Bead counts for each bacterial compartment at both time points for the wild-type and the PilCwt/PilT-null strains. The distribution of beads in the bacterial compartments is dramatically altered when tfp retraction occurs (wild type), but is stable in the absence of PilT-mediated retraction (PilCwt/PilT-null). Data represent mean±standard error of the mean.
Figure 6
Pilin immunoblotting at intimate adhesion time. At intimate adhesion time (8H), HUVEC monolayers infected with wild-type or PilCwt/PilT-null bacteria were scrapped and similar amounts of bacterial total protein extracts were probed for pilin by Western blot. Lane 1, wild type (Nm2C4.3); lane 2, PilCwt/PilT-null; lane 3, PilD-null strain (bacterial extract as control for pilin size, in which prepilin is not cleaved into mature pilin). Both wild-type and PilCwt/PilT-null strains show mature pilin patterns. Neither prepilin nor S-pilin could be seen.
Figure 7
De-biotinylation protection experiment on living bacteria. Bacteria were allowed to grow, and twitch in relevant case, in the presence of a biotinylation agent that could subsequently be cleaved off the bacterial surface under reducing conditions. Lane 1, wild type (Nm2C4.3); lane 2, PilCwt/PilT-null; lane 3, PilC-null/PilTwt; lane 4, PilE-null. Upper panel: Whole bacterial extracts were used in Western blots with HRP-coupled streptavidin in the search of biotinylated proteins that would be protected from surface de-biotinylation. The arrow indicates de-biotinylation-protected pilin, which is only observed in retraction-proficient strains. Lower panel: Biotinylated bands corresponding to pilin were identified by subsequent re-probing of the membrane with an anti-pilin antibody.
Figure 8
Model for the expression and retraction of neisserial tfp. Pilus extension: Tfp is formed in/along the cytoplasmic membrane (IM) by the assembly of mature pilin subunits that are stalled into the membrane, after processing by the prepilin peptidase PilD. The growing fibre is subsequently translocated to the bacterial surface through the outer membrane (OM) secretin PilQ. By homology with other machineries, the neisserial PilF is thought to have nucleotide-binding activities (Fernandez and Berenguer, 2000; Sandkvist, 2001). PilT-driven retraction is prevented as long as sufficient PilC proteins, which might act as a clip on the pilus, are expressed. Pilus retraction: Reduced amounts of PilC cannot prevent the action of PilT. Mature pilin subunits are thought to be translocated from the base of the fibre to the cytoplasmic membrane in an ATP-dependant process, thus leading to fibre retraction and accumulation of mature pilin along this membrane. Although not formally demonstrated for the neisserial machinery, reports in other species show that PilT is an ATPase and can form oligomers (Herdendorf et al, 2002; Okamoto and Ohmori, 2002). This model surmises that, when the PilT component is absent, fibres are normally assembled and extruded to the bacterial surface (situation in the left panel), but are not subjected to retraction. This results in the accumulation of tfp on the cell surface and bacteria appear hyperpiliated whatever the expression of PilC. The abolition of PilC in a PilTwt background allows increased retraction rates (situation in the right panel), and fibres are immediately retracted after expression on the bacterial surface, thus leading to poor piliation.
Similar articles
- Pilus-mediated adhesion of Neisseria meningitidis is negatively controlled by the pilus-retraction machinery.
Yasukawa K, Martin P, Tinsley CR, Nassif X. Yasukawa K, et al. Mol Microbiol. 2006 Jan;59(2):579-89. doi: 10.1111/j.1365-2958.2005.04954.x. Mol Microbiol. 2006. PMID: 16390451 - Pseudomonas aeruginosa Type IV pilus expression in Neisseria gonorrhoeae: effects of pilin subunit composition on function and organelle dynamics.
Winther-Larsen HC, Wolfgang MC, van Putten JP, Roos N, Aas FE, Egge-Jacobsen WM, Maier B, Koomey M. Winther-Larsen HC, et al. J Bacteriol. 2007 Sep;189(18):6676-85. doi: 10.1128/JB.00407-07. Epub 2007 Jun 15. J Bacteriol. 2007. PMID: 17573479 Free PMC article. - A systematic genetic analysis in Neisseria meningitidis defines the Pil proteins required for assembly, functionality, stabilization and export of type IV pili.
Carbonnelle E, Helaine S, Nassif X, Pelicic V. Carbonnelle E, et al. Mol Microbiol. 2006 Sep;61(6):1510-22. doi: 10.1111/j.1365-2958.2006.05341.x. Mol Microbiol. 2006. PMID: 16968224 - Type II secretion and type IV pili of Francisella.
Forsberg A, Guina T. Forsberg A, et al. Ann N Y Acad Sci. 2007 Jun;1105:187-201. doi: 10.1196/annals.1409.016. Epub 2007 Apr 13. Ann N Y Acad Sci. 2007. PMID: 17435117 Review. - Pulling together with type IV pili.
Nudleman E, Kaiser D. Nudleman E, et al. J Mol Microbiol Biotechnol. 2004;7(1-2):52-62. doi: 10.1159/000077869. J Mol Microbiol Biotechnol. 2004. PMID: 15170403 Review.
Cited by
- Pathogenesis of meningococcemia.
Coureuil M, Join-Lambert O, Lécuyer H, Bourdoulous S, Marullo S, Nassif X. Coureuil M, et al. Cold Spring Harb Perspect Med. 2013 Jun 1;3(6):a012393. doi: 10.1101/cshperspect.a012393. Cold Spring Harb Perspect Med. 2013. PMID: 23732856 Free PMC article. Review. - The biology of Neisseria adhesins.
Hung MC, Christodoulides M. Hung MC, et al. Biology (Basel). 2013 Jul 29;2(3):1054-109. doi: 10.3390/biology2031054. Biology (Basel). 2013. PMID: 24833056 Free PMC article. - Obstruction of pilus retraction stimulates bacterial surface sensing.
Ellison CK, Kan J, Dillard RS, Kysela DT, Ducret A, Berne C, Hampton CM, Ke Z, Wright ER, Biais N, Dalia AB, Brun YV. Ellison CK, et al. Science. 2017 Oct 27;358(6362):535-538. doi: 10.1126/science.aan5706. Science. 2017. PMID: 29074778 Free PMC article. - Chemotaxis Control of Transient Cell Aggregation.
Alexandre G. Alexandre G. J Bacteriol. 2015 Oct;197(20):3230-7. doi: 10.1128/JB.00121-15. Epub 2015 Jul 27. J Bacteriol. 2015. PMID: 26216846 Free PMC article. Review. - PilA localization affects extracellular polysaccharide production and fruiting body formation in Myxococcus xanthus.
Yang Z, Lux R, Hu W, Hu C, Shi W. Yang Z, et al. Mol Microbiol. 2010 Jun;76(6):1500-13. doi: 10.1111/j.1365-2958.2010.07180.x. Epub 2010 Apr 23. Mol Microbiol. 2010. PMID: 20444090 Free PMC article.
References
- Drake SL, Sandstedt SA, Koomey M (1997) PilP, a pilus biogenesis lipoprotein in Neisseria gonorrhoeae, affects expression of PilQ as a high-molecular-mass multimer. Mol Microbiol 23: 657–668 - PubMed
- Fernandez LA, Berenguer J (2000) Secretion and assembly of regular surface structures in Gram-negative bacteria. FEMS Microbiol Rev 24: 21–44 - PubMed
- Freitag NE, Seifert HS, Koomey M (1995) Characterization of the pilF-pilD pilus-assembly locus of Neisseria gonorrhoeae. Mol Microbiol 16: 575–586 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources