Hypoxia-inducible factor-1 (HIF-1) promotes its degradation by induction of HIF-alpha-prolyl-4-hydroxylases - PubMed (original) (raw)
Hypoxia-inducible factor-1 (HIF-1) promotes its degradation by induction of HIF-alpha-prolyl-4-hydroxylases
Jan H Marxsen et al. Biochem J. 2004.
Abstract
An important regulator involved in oxygen-dependent gene expression is the transcription factor HIF (hypoxia-inducible factor), which is composed of an oxygen-sensitive alpha-subunit (HIF-1alpha or HIF-2alpha) and a constitutively expressed beta-subunit. In normoxia, HIF-1alpha is destabilized by post-translational hydroxylation of Pro-564 and Pro-402 by a family of oxygen-sensitive dioxygenases. The three HIF-modifying human enzymes have been termed prolyl hydroxylase domain containing proteins (PHD1, PHD2 and PHD3). Prolyl hydroxylation leads to pVHL (von-Hippel-Lindau protein)-dependent ubiquitination and rapid proteasomal degradation of HIF-1alpha. In the present study, we report that human PHD2 and PHD3 are induced by hypoxia in primary and transformed cell lines. In the human osteosarcoma cell line, U2OS, selective suppression of HIF-1alpha expression by RNA interference resulted in a complete loss of hypoxic induction of PHD2 and PHD3. Induction of PHD2 by hypoxia was lost in pVHL-deficient RCC4 cells. These results suggest that hypoxic induction of PHD2 and PHD3 is critically dependent on HIF-alpha. Using a VHL capture assay, we demonstrate that HIF-alpha prolyl-4-hydroxylase capacity of cytoplasmic and nuclear protein extracts was enhanced by prolonged exposure to hypoxia. Degradation of HIF-1alpha after reoxygenation was accelerated, which demonstrates functional relevance of the present results. We propose a direct, negative regulatory mechanism, which limits accumulation of HIF-1alpha in hypoxia and leads to accelerated degradation on reoxygenation after long-term hypoxia.
Figures
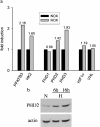
Figure 1. PHD induction in HepG2 cells by hypoxia
(a) Microarray analysis. Cells were incubated in an atmosphere of 1% O2 for 4 h. cDNA probes were generated as described in the Materials and methods section. Normoxic (NOX) and hypoxic (HOX) probes were combined and hybridized to the array overnight at 65 °C. Three replicate spots on each slide were averaged. (b) PHD2 Western blot. Cells were treated as for microarrays. Whole cell-protein extracts were subjected to SDS/PAGE and immunoblot analysis.
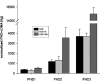
Figure 2. PHD induction in renal proximal tubule cells by hypoxia
Real-time quantitative RT–PCR was performed for PHD1, PHD2, PHD3 of normoxic (NOX), short-term hypoxic (HOX 1 h) and long-term hypoxic (HOX 18 h) RPTEC cultures. Bars indicate means±S.D. of three samples from one experiment. Results are given normalized to 60 S aRP mRNA as a housekeeping gene. These results are representative of two independent experiments.
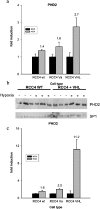
Figure 3. Normoxic and hypoxic PHD levels in VHL-deficient cells
Quantitative RT–PCR of different RCC4 sublines was performed. cDNA levels were corrected for 60 S aRP. Results are presented for (a) PHD2 and (c) PHD3 in wild-type (wt), mock-transfected (Va) and VHL-recompleted (VHL) RCC4 cells. Bars indicate means±S.D. of three independent samples from one experiment. (b) Samples were prepared from three independent cultures of both cell lines in normoxia and in hypoxia and analysed for PHD2 and SP1 protein expression. SP1 serves as a loading and transfer control.
Figure 4. Western-blot analysis of HIF-α in U2OS cells
(a) Western-blot analysis of HIF-1α protein levels in U2OS cells treated with siRNA oligonucleotides. Cells were incubated with scramble or HIF-1α or HIF-2α oligonucleotides for 4 h and then subjected to hypoxia for 18 h. (b) Western-blot analysis of HIF-2α after 4 h of hypoxia demonstrating hypoxic induction of HIF-2α in VHL-transfected 786–0 cells but not in U2OS cells.
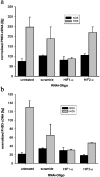
Figure 5. PHD expression in normoxia and in hypoxia after RNAi
Real-time quantitative RT–PCR for (a) PHD2 and (b) PHD3 in U2OS cultures transfected with the indicated siRNA oligonucleotides. Bars indicate the normalized means±S.D. of three samples from one experiment.
Figure 6. In vitro hydroxylase activity assay
Equal amounts of nuclear and cytoplasmic protein extracts from normoxic (N), short-term hypoxic (H, 1 h) and long-term hypoxic (H, 18 h) U2OS cultures hydroxylated Gal-HIF-1α549–582 bound to agarose beads. After stopping hydroxylation by adding desferrioxamine, 35S-labelled IVTT-pVHL was added and incubated overnight. Supernatants were subjected to SDS/PAGE and 35S-pVHL was detected by autoradiography. Captured 35S-VHL indicates hydroxylase activity of the protein extracts modifying the HIF-ODD. IVTT-pVHL appears in two isoforms due to an internal in-frame start codon. ‘neg.’ and ‘pos.’ indicate hydroxylase activity of unprogrammed reticulocyte lysate and IVTT-PHD2 respectively.
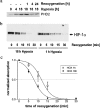
Figure 7. Western-blot analysis of PHD2 and HIF-1α after hypoxic incubation and reoxygenation
U2OS cultures were lysed at the indicated time of hypoxia and reoxygenation after long-term (18 h) and short-term (1 h) hypoxia. SDS/PAGE was performed with a total of 40 μg protein loaded in each lane: (a) PHD2; (b) time course of HIF-1α degradation; (c) line plot showing levels of HIF-1α protein at the time points shown in (b). Data originate from four repetitions of the hypoxia/reoxygenation experiments. HIF-1α bands were analysed by densitometry; results are expressed as means±S.D. Lines were calculated by non-linear regression.
Similar articles
- Regulation of HIF prolyl hydroxylases by hypoxia-inducible factors.
Aprelikova O, Chandramouli GV, Wood M, Vasselli JR, Riss J, Maranchie JK, Linehan WM, Barrett JC. Aprelikova O, et al. J Cell Biochem. 2004 Jun 1;92(3):491-501. doi: 10.1002/jcb.20067. J Cell Biochem. 2004. PMID: 15156561 - HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia.
Berra E, Benizri E, Ginouvès A, Volmat V, Roux D, Pouysségur J. Berra E, et al. EMBO J. 2003 Aug 15;22(16):4082-90. doi: 10.1093/emboj/cdg392. EMBO J. 2003. PMID: 12912907 Free PMC article. - Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor.
Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM. Appelhoff RJ, et al. J Biol Chem. 2004 Sep 10;279(37):38458-65. doi: 10.1074/jbc.M406026200. Epub 2004 Jul 7. J Biol Chem. 2004. PMID: 15247232 - Von Hippel-Lindau tumor suppressor protein and hypoxia-inducible factor in kidney cancer.
Maynard MA, Ohh M. Maynard MA, et al. Am J Nephrol. 2004 Jan-Feb;24(1):1-13. doi: 10.1159/000075346. Epub 2003 Dec 3. Am J Nephrol. 2004. PMID: 14654728 Review. - Regulation of HIF by the von Hippel-Lindau tumour suppressor: implications for cellular oxygen sensing.
Mole DR, Maxwell PH, Pugh CW, Ratcliffe PJ. Mole DR, et al. IUBMB Life. 2001 Jul;52(1-2):43-7. doi: 10.1080/15216540252774757. IUBMB Life. 2001. PMID: 11795592 Review.
Cited by
- Hypoxia-Inducible Histone Lysine Demethylases: Impact on the Aging Process and Age-Related Diseases.
Salminen A, Kaarniranta K, Kauppinen A. Salminen A, et al. Aging Dis. 2016 Mar 15;7(2):180-200. doi: 10.14336/AD.2015.0929. eCollection 2016 Mar. Aging Dis. 2016. PMID: 27114850 Free PMC article. Review. - Role of prolyl hydroxylase domain proteins in bone metabolism.
Wolf D, Muralidharan A, Mohan S. Wolf D, et al. Osteoporos Sarcopenia. 2022 Mar;8(1):1-10. doi: 10.1016/j.afos.2022.03.001. Epub 2022 Mar 22. Osteoporos Sarcopenia. 2022. PMID: 35415275 Free PMC article. Review. - Coxiella burnetii Affects HIF1α Accumulation and HIF1α Target Gene Expression.
Hayek I, Szperlinski M, Lührmann A. Hayek I, et al. Front Cell Infect Microbiol. 2022 Jun 9;12:867689. doi: 10.3389/fcimb.2022.867689. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35755850 Free PMC article. - Self-Sustained Regulation or Self-Perpetuating Dysregulation: ROS-dependent HIF-YAP-Notch Signaling as a Double-Edged Sword on Stem Cell Physiology and Tumorigenesis.
Guo CL. Guo CL. Front Cell Dev Biol. 2022 Jun 14;10:862791. doi: 10.3389/fcell.2022.862791. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35774228 Free PMC article. Review. - Neurogenic and pericytic plasticity of conditionally immortalized cells derived from renal erythropoietin-producing cells.
Bapst AM, Knöpfel T, Nolan KA, Imeri F, Schuh CD, Hall AM, Guo J, Katschinski DM, Wenger RH. Bapst AM, et al. J Cell Physiol. 2022 May;237(5):2420-2433. doi: 10.1002/jcp.30677. Epub 2022 Jan 10. J Cell Physiol. 2022. PMID: 35014036 Free PMC article.
References
- Wenger R. H. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002;16:1151–1162. - PubMed
- Semenza G. L. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr. Opin. Genet. Dev. 1998;8:588–594. - PubMed
- Masson N., Ratcliffe P. J. HIF prolyl and asparaginyl hydroxylases in the biological response to intracellular O2 levels. J. Cell Sci. 2003;116:3041–3049. - PubMed
- Ivan M., Kondo K., Yang H., Kim W., Valiando J., Ohh M., Salic A., Asara J. M., Lane W. S., Kaelin W. G., Jr HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. - PubMed
- Jaakkola P., Mole D. R., Tian Y. M., Wilson M. I., Gielbert J., Gaskell S. J., Kriegsheim A., Hebestreit H. F., Mukherji M., Schofield C. J., et al. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources