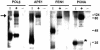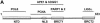XRCC1 co-localizes and physically interacts with PCNA - PubMed (original) (raw)
. 2004 Apr 23;32(7):2193-201.
doi: 10.1093/nar/gkh556. Print 2004.
Affiliations
- PMID: 15107487
- PMCID: PMC407833
- DOI: 10.1093/nar/gkh556
XRCC1 co-localizes and physically interacts with PCNA
Jinshui Fan et al. Nucleic Acids Res. 2004.
Abstract
X-ray Repair Cross Complementing 1 (XRCC1) is thought to function as a scaffolding protein in both base excision repair and single-strand break repair (SSBR), since it interacts with several proteins participating in these related pathways and has no known enzymatic activity. Moreover, studies indicate that XRCC1 possesses discrete G1 and S phase-specific functions. To further define the contribution of XRCC1 to DNA metabolism, we determined the in vivo localization pattern of this protein and searched for novel protein interactors. We report here that XRCC1 co-localizes with proliferating cell nuclear antigen (PCNA) at DNA replication foci, observed exclusively in the S phase of undamaged HeLa cells. Furthermore, fluorescence resonance energy transfer (FRET) analysis and co-immunoprecipitation indicate that XRCC1 and PCNA are in a complex and likely physically interact in vivo. In vitro biochemical analysis demonstrated that these two proteins associate directly, with the interaction being mediated by residues between amino acids 166 and 310 of XRCC1. The current evidence suggests a model where XRCC1 is sequestered via its interaction with PCNA to sites of DNA replication factories to facilitate efficient SSBR in S phase.
Figures
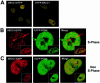
Figure 1
Localization of XRCC1 and PCNA in HeLa cells. (A) XRCC1 forms discrete foci. Constructs expressing either C- (left) or N-terminal (right) YFP-tagged human XRCC1 were transfected into HeLa cells. Fluorescently tagged protein was visualized using a Zeiss LSM 510 laser scanning microscope equipped with a Plan-Apochromate 63×/1.4 oil immersion objective (see Materials and Methods). (B) XRCC1 and PCNA co-localize in S phase human cells. The C-terminal YFP-tagged XRCC1 expression construct was co-transfected with a ECFP–PCNA expression construct and S phase cells with PCNA foci (which comprised ∼10–20% of the cell population) were examined as above. The green arrow indicates foci with a higher degree of PCNA relative to XRCC1 and the orange arrows indicate foci with a higher level of XRCC1. (C) XRCC1 forms foci in non-S phase cells. The same constructs as in (B) were co-transfected and the cellular distribution of XRCC1 and PCNA was evaluated. Insets in (B) and (C) show a separate, representative cell.
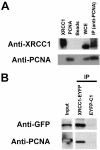
Figure 2
Co-immunoprecipitation of XRCC1 and PCNA. (A) Anti-PCNA antibody pulls down XRCC1. Whole cell extracts prepared from human 293T cells were subject to immunoprecipitation with anti-PCNA antibody as described in Materials and Methods. The precipitated proteins (IP) were fractionated by SDS–PAGE and probed with either anti-XRCC1 or anti-PCNA antibodies (as indicated) using standard immunoblotting techniques. XRCC1, purified recombinant XRCC1; PCNA, purified recombinant PCNA; beads, agarose bead control, without PCNA antibody; WCE, whole cell extract (∼30 µg); IP, immunoprecipitant. (B) Anti-GFP antibody pulls down XRCC1–EYFP and PCNA. Cell extracts were prepared from HeLa cells stably expressing either XRCC1–EYFP or EYFP alone (EYFP-C1) and immunoprecipitations were performed with anti-GFP as described in Materials and Methods. The precipitated proteins (IP) were analyzed as above with the indicated antibodies. Input was 20% of the starting material from the XRCC1–EYFP extracts. Note that GFP alone is not shown, as it migrated at a much lower molecular weight than the XRCC1–EYFP fusion protein.
Figure 3
XRCC1 physically interacts with PCNA in vitro. Purified recombinant XRCC1 protein (+ lanes) was examined for physical association with purified recombinant POLβ, APE1, FEN1 or PCNA (as indicated) using the in vitro protein interaction assay described in Materials and Methods. Non-specific interactions of the four recombinant proteins with the affinity matrix were examined in the absence of XRCC1 (– lanes). Matrix bound proteins were analyzed by SDS–PAGE and silver staining (representative gel shown). I indicates the initial input for the POLβ, APE1, FEN1 and PCNA proteins. The asterisk denotes the location of the PCNA protein and the arrow indicates the position of the HIS- and S-tagged recombinant XRCC1 protein. Molecular weight protein standards are shown (in kDa) to the right.
Figure 4
PCNA interacts with amino acids between 166 and 310 of XRCC1. (A) Schematic of the five human XRCC1 protein fragments. Names of protein fragments and the amino acid regions covered are indicated (see also Table 1). The locations of known functional domains and consensus sequences are denoted. XRCC1_N, N-terminal DNA binding and POLβ interaction domain; NLS, nuclear localization signals; BRCT1 and BRCT2, the central and C-terminal BRCT domains. (B) Purified XRCC1 recombinant proteins. Nickel-purified XRCC1 proteins were subjected to SDS–PAGE and stained with Coomassie brilliant blue. Full-length XRCC1 and protein fragments are indicated. Molecular weight protein standards (in kDa) are shown to the right. (C) PCNA interacts with the XNTD and MD fragments of XRCC1. Recombinant full-length XRCC1 protein (lane 1) or one of the XRCC1 fragments, NTD (lane 2), XNTD (lane 3), MD (lane 4), BLB (lane 5) and BRCT2 (lane 6), was bound to a Ni affinity matrix. Following incubation with purified PCNA (see Materials and Methods), matrix-associated proteins were separated by SDS–PAGE, transferred to a PVDF membrane and probed with anti-PCNA antibody. Lanes 7 (affinity matrix, with no XRCC1 protein) and 8 (150 ng purified PCNA) represent the negative and positive controls, respectively.
Figure 5
(A) Schematic of XRCC1 interacting regions and protein partners. Thus far, at least eight proteins (see text for details), including PCNA described in this work, have been found to directly interact with XRCC1. The regions that are responsible for these interactions, excluding PNK, have been assigned (see diagram). Note that TDP1 has not been shown to directly associate with XRCC1. (B) A model for ‘replication-coupled repair’. XRCC1 is linked to DNA replication factories (i.e. foci) via its interaction with PCNA. This interaction increases the local concentration of XRCC1 (and potentially PARP-1) and facilitates rapid recognition and processing of SSBs, as XRCC1 functions to recruit other factors for repair. In other words, the coordination between replication and XRCC1 ensures proficient repair of SSBs and prevents DSB formation via damage-induced replication fork collapse and homologous recombination. Two other replication-linked pathways, namely post-replicative mismatch repair (MMR) and BER, operate on the newly synthesized strand behind the replication fork (see related reviews –53). Also shown is lagging strand ligation.
Figure 5
(A) Schematic of XRCC1 interacting regions and protein partners. Thus far, at least eight proteins (see text for details), including PCNA described in this work, have been found to directly interact with XRCC1. The regions that are responsible for these interactions, excluding PNK, have been assigned (see diagram). Note that TDP1 has not been shown to directly associate with XRCC1. (B) A model for ‘replication-coupled repair’. XRCC1 is linked to DNA replication factories (i.e. foci) via its interaction with PCNA. This interaction increases the local concentration of XRCC1 (and potentially PARP-1) and facilitates rapid recognition and processing of SSBs, as XRCC1 functions to recruit other factors for repair. In other words, the coordination between replication and XRCC1 ensures proficient repair of SSBs and prevents DSB formation via damage-induced replication fork collapse and homologous recombination. Two other replication-linked pathways, namely post-replicative mismatch repair (MMR) and BER, operate on the newly synthesized strand behind the replication fork (see related reviews –53). Also shown is lagging strand ligation.
Similar articles
- PARP inhibition versus PARP-1 silencing: different outcomes in terms of single-strand break repair and radiation susceptibility.
Godon C, Cordelières FP, Biard D, Giocanti N, Mégnin-Chanet F, Hall J, Favaudon V. Godon C, et al. Nucleic Acids Res. 2008 Aug;36(13):4454-64. doi: 10.1093/nar/gkn403. Epub 2008 Jul 4. Nucleic Acids Res. 2008. PMID: 18603595 Free PMC article. - The region of XRCC1 which harbours the three most common nonsynonymous polymorphic variants, is essential for the scaffolding function of XRCC1.
Hanssen-Bauer A, Solvang-Garten K, Gilljam KM, Torseth K, Wilson DM 3rd, Akbari M, Otterlei M. Hanssen-Bauer A, et al. DNA Repair (Amst). 2012 Apr 1;11(4):357-66. doi: 10.1016/j.dnarep.2012.01.001. Epub 2012 Jan 26. DNA Repair (Amst). 2012. PMID: 22281126 Free PMC article. - Direct interaction between XRCC1 and UNG2 facilitates rapid repair of uracil in DNA by XRCC1 complexes.
Akbari M, Solvang-Garten K, Hanssen-Bauer A, Lieske NV, Pettersen HS, Pettersen GK, Wilson DM 3rd, Krokan HE, Otterlei M. Akbari M, et al. DNA Repair (Amst). 2010 Jul 1;9(7):785-95. doi: 10.1016/j.dnarep.2010.04.002. Epub 2010 May 13. DNA Repair (Amst). 2010. PMID: 20466601 Free PMC article. - Colocalization of human Rad17 and PCNA in late S phase of the cell cycle upon replication block.
Dahm K, Hübscher U. Dahm K, et al. Oncogene. 2002 Oct 31;21(50):7710-9. doi: 10.1038/sj.onc.1205872. Oncogene. 2002. PMID: 12400013 - X-ray repair cross complementing protein 1 in base excision repair.
Hanssen-Bauer A, Solvang-Garten K, Akbari M, Otterlei M. Hanssen-Bauer A, et al. Int J Mol Sci. 2012 Dec 17;13(12):17210-29. doi: 10.3390/ijms131217210. Int J Mol Sci. 2012. PMID: 23247283 Free PMC article. Review.
Cited by
- Human base excision repair complex is physically associated to DNA replication and cell cycle regulatory proteins.
Parlanti E, Locatelli G, Maga G, Dogliotti E. Parlanti E, et al. Nucleic Acids Res. 2007;35(5):1569-77. doi: 10.1093/nar/gkl1159. Epub 2007 Feb 8. Nucleic Acids Res. 2007. PMID: 17289756 Free PMC article. - An 8-oxo-guanine repair pathway coordinated by MUTYH glycosylase and DNA polymerase lambda.
van Loon B, Hübscher U. van Loon B, et al. Proc Natl Acad Sci U S A. 2009 Oct 27;106(43):18201-6. doi: 10.1073/pnas.0907280106. Epub 2009 Oct 9. Proc Natl Acad Sci U S A. 2009. PMID: 19820168 Free PMC article. - XRCC1 is required for DNA single-strand break repair in human cells.
Brem R, Hall J. Brem R, et al. Nucleic Acids Res. 2005 May 2;33(8):2512-20. doi: 10.1093/nar/gki543. Print 2005. Nucleic Acids Res. 2005. PMID: 15867196 Free PMC article. - JWA regulates XRCC1 and functions as a novel base excision repair protein in oxidative-stress-induced DNA single-strand breaks.
Wang S, Gong Z, Chen R, Liu Y, Li A, Li G, Zhou J. Wang S, et al. Nucleic Acids Res. 2009 Apr;37(6):1936-50. doi: 10.1093/nar/gkp054. Epub 2009 Feb 10. Nucleic Acids Res. 2009. PMID: 19208635 Free PMC article. - Mammalian DNA ligases; roles in maintaining genome integrity.
Sallmyr A, Bhandari SK, Naila T, Tomkinson AE. Sallmyr A, et al. J Mol Biol. 2024 Jan 1;436(1):168276. doi: 10.1016/j.jmb.2023.168276. Epub 2023 Sep 13. J Mol Biol. 2024. PMID: 37714297 Free PMC article. Review.
References
- Thompson L.H., Brookman,K.W., Dillehay,L.E., Carrano,A.V., Mazrimas,J.A., Mooney,C.L. and Minkler,J.L. (1982) A CHO-cell strain having hypersensitivity to mutagens, a defect in DNA strand-break repair and an extraordinary baseline frequency of sister-chromatid exchange. Mutat. Res., 95, 427–440. - PubMed
- Dillehay L.E., Thompson,L.H. and Carrano,A.V. (1984) DNA-strand breaks associated with halogenated pyrimidine incorporation. Mutat. Res., 131, 129–136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous