Memory traces of trace memories: neurogenesis, synaptogenesis and awareness - PubMed (original) (raw)
Review
Memory traces of trace memories: neurogenesis, synaptogenesis and awareness
Tracey J Shors. Trends Neurosci. 2004 May.
Abstract
To associate events that are disparate in time, the brain must record, retain and perhaps even reflect on the individual events themselves. Aspects of such learning can be probed with trace conditioning, during which an animal learns to associate events that are temporally distant from one another. For decades, we have known that the formation of so-called trace memories (in which one stimulus is associated with a second stimulus that is discontinuous and later in time) depends on the hippocampal formation. Recent findings indicate that the hippocampus is crucial for the initial acquisition of trace memories but not for their expression or long-term storage. More recent findings implicate neurogenesis, synaptogenesis and awareness in the formation of trace memories.
Figures
Figure 1
Temporal relationships between stimulus events mediate the ability to acquire associative memories. The conditioned stimulus (CS) is depicted in blue and followed by an unconditioned stimulus (US) in yellow. (a) Delay conditioning is represented as two stimuli that immediately follow one another. The stimuli can also overlap in time as long as the US follows the CS. In either case, the association is usually acquired rapidly, is not associated with awareness and, as such, is considered a procedural memory. (b) Trace conditioning is represented as two stimuli that are not contiguous in time. The stimuli are typically separated from one another by a trace interval, often referred to as the ‘gap’. This association is more difficult to learn and because of its dependence on the hippocampal formation [3,13,14] and association with awareness is often considered a declarative memory [51]. (c) Explicitly unpaired stimuli are presented one after another but the time between stimuli is random and unpredictable. After repeated exposure to these stimuli, an animal learns that the CS is followed by a US but does not know when the US will occur other than that it will not occur during the CS, and so the animal does not display excitatory responses to the CS. As illustrated, the stimulus events and their relationships to one another are remarkably similar between trace and unpaired conditioning, emphasizing the amazing ability of animals to detect predictive relationships among stimuli as they encounter them in their environment.
Figure 2
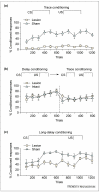
Formation of trace memories requires an intact hippocampus. (a) Eye-blinks that were detected during the temporal gap were considered conditioned responses and are represented as a percentage of responses during training. As shown, animals with excitotoxic lesions of the hippocampus emitted virtually no conditioned responses even after > 1000 trials of trace eyeblink training [14]. (b) However, animals with lesions to the hippocampus can learn the conditioned response when a delay training protocol is used. In this experiment, the intervals between the conditioned stimulus (CS) and the unconditioned stimulus (US) were the same except that the CS was temporally contiguous and overlapped slightly with the US. After training with delay conditioning (dashed line), animals with hippocampal lesions could perform the trace conditioning task. (c) As the stimulus relationships become more difficult to learn, the hippocampus becomes involved. Using a long-delay paradigm in which the CS and the US are continuous in time but the time between stimuli onsets is extended, animals with lesions to the hippocampus are learning-impaired. These animals eventually learned and, moreover, were able to perform the trace conditioned response by moving the response to accommodate the new onset time of the US (data not shown) [14]. All three panels were generated using data from Ref. [14].
Figure 3
Formation of trace memories is impaired when the population of newly generated neurons is depleted. (a) (i) New cells in the hippocampus were labeled with bromodeoxyuridine (BrdU), a thymidine analog that labels into dividing cells [56]. Animals received daily injections of either an anti-mitotic agent (MAM) or saline for two weeks. The number of cells labeled after treatment for groups injected with either MAM or saline is presented here as group averages. Using neuron specific markers, it was determined that the vast majority of BrdU-labeled cells were neurons. (ii) The percentage of conditioned (learned) eyeblink responses during training. Animals treated with MAM emitted many fewer learned responses during trace conditioning than during delay eyeblink conditioning [41]. Asterisk indicates a significantly different result. Using data from Ref. [41]. (b) Fear conditioning is represented as a decrease in movement during the trace interval after repeated presentations of a tone conditioned stimulus (CS) and a footshock unconditioned stimulus (US). After two weeks of treatment with MAM and corresponding depletion of the pool of new neurons, animals expressed much less fear (more movement) during the trace interval, and showed reduced anticipation of the US [42]. Thus, their ability to learn the trace fear response was impaired. Asterisks indicate significantly different results. Using data from Ref. [42]. (c) Latency to reach the platform during training on the spatial navigation version of the Morris water maze. As in (a) and (b), rats were treated with MAM or saline for two weeks and then trained. With very few new neurons, the animals readily learned the spatial navigation task [42]. These results are consistent with a recent study showing that nearly complete elimination of adult-generated cells does not alter spatial maze learning [43]. Using data from Ref. [42].
Figure 4
The formation of trace memories increases the survival of adult-generated neurons. (a) The dentate gyrus of the hippocampal formation (i) and new cells within the granule cell layer (ii) that were labeled with bromodeoxyuridine (BrdU). (b) Animals were injected with one dose of BrdU to label cells generated in the dentate gyrus of the hippocampal formation [36]. One week later they were trained on trace or delay eyeblink conditioning or exposed to unpaired stimuli. One day after training, the number of cells, the vast majority of which were neurons, was increased in animals that were exposed to a trace conditioning paradigm (Figure 1b) relative to those exposed to delay conditioning (Figure 1a) or explicitly unpaired stimuli (Figure 1c). Thus, the formation of trace memories preferentially rescued neurons in the adult hippocampus from death. Asterisk indicates a significantly different result. Using data from Ref. [36].
Similar articles
- Inhibition of hippocampal protein synthesis following recall disrupts expression of episodic-like memory in trace conditioning.
Runyan JD, Dash PK. Runyan JD, et al. Hippocampus. 2005;15(3):333-9. doi: 10.1002/hipo.20055. Hippocampus. 2005. PMID: 15523611 - Goldfish hippocampal pallium is essential to associate temporally discontiguous events.
Rodríguez-Expósito B, Gómez A, Martín-Monzón I, Reiriz M, Rodríguez F, Salas C. Rodríguez-Expósito B, et al. Neurobiol Learn Mem. 2017 Mar;139:128-134. doi: 10.1016/j.nlm.2017.01.002. Epub 2017 Jan 6. Neurobiol Learn Mem. 2017. PMID: 28065713 - Associative learning elicits the formation of multiple-synapse boutons.
Geinisman Y, Berry RW, Disterhoft JF, Power JM, Van der Zee EA. Geinisman Y, et al. J Neurosci. 2001 Aug 1;21(15):5568-73. doi: 10.1523/JNEUROSCI.21-15-05568.2001. J Neurosci. 2001. PMID: 11466428 Free PMC article. - Brain plasticity mechanisms and memory: a party of four.
Bruel-Jungerman E, Davis S, Laroche S. Bruel-Jungerman E, et al. Neuroscientist. 2007 Oct;13(5):492-505. doi: 10.1177/1073858407302725. Neuroscientist. 2007. PMID: 17901258 Review. - Retrieval of emotional memories.
Buchanan TW. Buchanan TW. Psychol Bull. 2007 Sep;133(5):761-79. doi: 10.1037/0033-2909.133.5.761. Psychol Bull. 2007. PMID: 17723029 Free PMC article. Review.
Cited by
- Intertrial unconditioned stimuli differentially impact trace conditioning.
Williams DA, Todd TP, Chubala CM, Ludvig EA. Williams DA, et al. Learn Behav. 2017 Mar;45(1):49-61. doi: 10.3758/s13420-016-0240-3. Learn Behav. 2017. PMID: 27495932 - Temporal-difference reinforcement learning with distributed representations.
Kurth-Nelson Z, Redish AD. Kurth-Nelson Z, et al. PLoS One. 2009 Oct 20;4(10):e7362. doi: 10.1371/journal.pone.0007362. PLoS One. 2009. PMID: 19841749 Free PMC article. - Deletion of lysophosphatidic acid receptor LPA1 reduces neurogenesis in the mouse dentate gyrus.
Matas-Rico E, García-Diaz B, Llebrez-Zayas P, López-Barroso D, Santín L, Pedraza C, Smith-Fernández A, Fernández-Llebrez P, Tellez T, Redondo M, Chun J, De Fonseca FR, Estivill-Torrús G. Matas-Rico E, et al. Mol Cell Neurosci. 2008 Nov;39(3):342-55. doi: 10.1016/j.mcn.2008.07.014. Epub 2008 Jul 29. Mol Cell Neurosci. 2008. PMID: 18708146 Free PMC article. - Significant life events and the shape of memories to come: a hypothesis.
Shors TJ. Shors TJ. Neurobiol Learn Mem. 2006 Mar;85(2):103-15. doi: 10.1016/j.nlm.2005.09.004. Epub 2005 Nov 10. Neurobiol Learn Mem. 2006. PMID: 16289750 Free PMC article. - Covariance modeling of MRI brain volumes in memory circuitry in schizophrenia: Sex differences are critical.
Abbs B, Liang L, Makris N, Tsuang M, Seidman LJ, Goldstein JM. Abbs B, et al. Neuroimage. 2011 Jun 15;56(4):1865-74. doi: 10.1016/j.neuroimage.2011.03.079. Epub 2011 Apr 8. Neuroimage. 2011. PMID: 21497198 Free PMC article.
References
- Pavlov I. Conditioned Reflexes. Oxford University Press; 1927. pp. 197–275.
- Gormezano I, et al. Twenty years of classical conditioning research with the rabbit. In: Praque JM, Epstein AN, editors. Progress in Psychobiology and Physiological Psychology. Academic; New York: 1983.
- Weiss C, et al. Hippocampal lesions prevent trace eyeblink conditioning in the freely moving rat. Behav. Brain Res. 1999;99:123–132. - PubMed
- McEchron MD, et al. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8:638–646. - PubMed
- McEchron M, et al. Neurotoxic lesions of the dorsal hippocampus disrupt auditory-cued trace heart (fear) conditioning in rabbits. Hippocampus. 2000;10:739–751. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources