Trans-endocytosis via spinules in adult rat hippocampus - PubMed (original) (raw)
Trans-endocytosis via spinules in adult rat hippocampus
Josef Spacek et al. J Neurosci. 2004.
Abstract
Locations of a distinctive mode of trans-endocytosis involving dendrites, axons, and glia were quantified through serial section electron microscopy. Short vesicular or long vermiform evaginations emerged from dendrites and axons and were engulfed by presynaptic or neighboring axons, astrocytes, and, surprisingly, a growth cone to form double-membrane structures called spinules. In total, 254 spinules were evaluated in 326 microm(3) of stratum radiatum in area CA1 of mature rat hippocampus. Spinules emerged from spine heads (62%), necks (24%), axons (13%), dendritic shafts (1%), or nonsynaptic protrusions (<1%) and invaginated into axons (approximately 90%), astrocytic processes (approximately 8%), or a growth cone (approximately 1%). Coated pits occurred on the engulfing membrane at the tips of most spinules (69%), and double-membrane structures occurred freely in axonal and astrocytic cytoplasm, suggesting trans-endocytosis. Spinule locations differed among mushroom and thin spines. For mushroom spines, most (84%) of the spinules were engulfed by presynaptic axons, 16% by neighboring axons, and none by astrocytic processes. At thin spines, only 17% of the spinules were engulfed by presynaptic axons, whereas 67% were engulfed by neighboring axons and 14% by astrocytic processes. Spinules engulfed by astrocytic processes support the growing evidence that perisynaptic glia interact directly with synapses at least on thin spines. Spinules with neighboring axons may provide a mechanism for synaptic competition in the mature brain. Trans-endocytosis of spinules by presynaptic axons suggest retrograde signaling or coordinated remodeling of presynaptic and postsynaptic membranes to remove transient perforations and assemble the postsynaptic density of large synapses on mushroom spines.
Figures
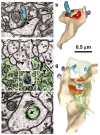
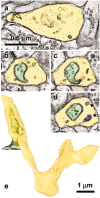
Figure 1.
Spinules on mushroom dendritic spines. a, Micrograph through the center of a spinule (turquoise) emerging from a perforation (arrowhead) in the postsynaptic density into the presynaptic axon. b, Reconstruction of the spine illustrated in a with the spinule in turquoise and the PSD surface area in red. c, d, Serial sections through the presynaptic axon (pre; green) and a spinule (arrowhead; turquoise) emerging from the edge of a mushroom spine head into the presynaptic axon and another spinule also emerging from the edge of the spine head (arrowhead; lavender) but invaginating a neighboring axon (n). e, High magnification of serial section beyond d showing a coating along the cytoplasmic surface of the spinule on the side of the invaginated presynaptic axon (arrowhead). f, Later sections of the mushroom spine head showing where the presynaptic axon deeply invaginated the spine head in a vesicle-free zone adjacent to a cell-adhesion (arrow) that is adjacent to the postsynaptic density on subsequent serial sections. g, Three-dimensional reconstruction of the mushroom spine (beige) with perforated synapse (red) and several small spinules into the presynaptic axon (turquoise spinules) or neighboring axon (lavender spinules).
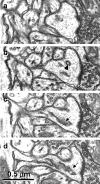
Figure 2.
Serial sections through a mushroom spine show the clear distinction between single membrane-coated vesicles (b, arrowhead), the smooth endoplasmic reticulum of a spine apparatus (c, d, arrows), and the spinules illustrated in Figure 1.
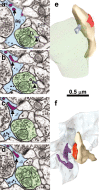
Figure 3.
Spinules from thin spine heads into a neighboring axon or an astrocytic process. a, b, Serial sections though a small spinule (arrowhead; lavender) emerging from a thin spine head into a neighboring axon (n; green). In serial sections _a_-d, portions of a long spinule (arrow; purple) emerge from a thin spine head into an astrocytic process (as, light blue). e, Reconstruction of the small spinule (lavender) from the thin spine neck into the neighboring axon (green) as illustrated in a and b; postsynaptic density on the spine is red. f, Reconstruction of a thin spine with long spinule emerging from its head (purple) into an astrocytic process (light blue) as illustrated in _a_-d.
Figure 4.
Spinules from thin and branched spine necks into neighboring axons. a, Lavender spinule (arrow) from thin spine neck into neighboring axon (green) is reconstructed in b. c, Lavender spinule with coated tip (arrow) from a branched spine neck into a neighboring axon (green) is reconstructed in d.
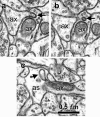
Figure 5.
Spinules between neighboring axons and between axons and astrocytic process. a, Origin of spinule (arrow) emerging from the left axon (ax) into a neighboring axon (ax). b, Adjacent serial section with a illustrating the coat at the tip of the axonal spinule. c, Spinule emerging from an axon (ax; arrow) into an astrocyte (as).
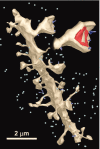
Figure 6.
Growth cone in mature hippocampus receives spinules from an axon. a, The lamellar junction (yellow) between two branch points shows numerous tubulo-vesicular components characteristic of growth cones. _b_-d, Serial sections through one of the spinules emerging from an axon (green) that has a coated tip on the cytoplasmic side of the growth cone invagination (d, arrow) and that is distinguished from a single membrane-coated vesicle (d, arrowhead). e, Reconstruction of the growth cone (yellow) and axon (green) that forms spinules with it. Scale bar in a applies to _a_-d.
Figure 7.
Reconstructed locations of spinules (dark blue protrusions) on a dendrite and enlarged spine. Other spinules in the surrounding neuropil are marked with light blue spheres to give a visual guide for their relative densities.
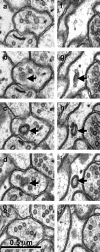
Figure 8.
_a_-j, Serial sections through free double-walled vesicles (arrows) in an axonal bouton (_a_-e) and an astrocytic process (_f_-j). Sections at the beginning and end of each series illustrate that these apparent spinules are not connected to neighboring structures but are free within the cytoplasm of the axon or astrocyte.
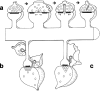
Figure 9.
Models of spinule functions. a, Process whereby trans-endocytosis of spinules removes excess presynaptic and postsynaptic plasma membrane after substantial activation results in transient perforated or segmented synapses on mushroom spines. This process could also provide retrograde signaling. b, Neighboring axons vying for synapses via spinules on the necks and heads of thin spines. c, Intercellular signaling between thin spines and perisynaptic glia via spinules.
Similar articles
- Three-dimensional organization of cell adhesion junctions at synapses and dendritic spines in area CA1 of the rat hippocampus.
Spacek J, Harris KM. Spacek J, et al. J Comp Neurol. 1998 Mar 30;393(1):58-68. doi: 10.1002/(sici)1096-9861(19980330)393:1<58::aid-cne6>3.0.co;2-p. J Comp Neurol. 1998. PMID: 9520101 - Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat.
Spacek J, Harris KM. Spacek J, et al. J Neurosci. 1997 Jan 1;17(1):190-203. doi: 10.1523/JNEUROSCI.17-01-00190.1997. J Neurosci. 1997. PMID: 8987748 Free PMC article. - Critical assessment of the involvement of perforations, spinules, and spine branching in hippocampal synapse formation.
Sorra KE, Fiala JC, Harris KM. Sorra KE, et al. J Comp Neurol. 1998 Aug 24;398(2):225-40. J Comp Neurol. 1998. PMID: 9700568 - Dendritic spinule-mediated structural synaptic plasticity: Implications for development, aging, and psychiatric disease.
Zaccard CR, Gippo I, Song A, Geula C, Penzes P. Zaccard CR, et al. Front Mol Neurosci. 2023 Jan 20;16:1059730. doi: 10.3389/fnmol.2023.1059730. eCollection 2023. Front Mol Neurosci. 2023. PMID: 36741924 Free PMC article. Review. - [Three-dimentional organization of synapses and astroglia in the hippocampus of rats and ground squirrels: new structural and functional paradigms of the synapse function].
Popov VI, Medvedev NI, Rogachevskiĭ VV, Ignat'ev DA, Stewart MG, Fesenko EE. Popov VI, et al. Biofizika. 2003 Mar-Apr;48(2):289-308. Biofizika. 2003. PMID: 12723356 Review. Russian.
Cited by
- Synaptic spinules are reliable indicators of excitatory presynaptic bouton size and strength and are ubiquitous components of excitatory synapses in CA1 hippocampus.
Gore A, Yurina A, Yukevich-Mussomeli A, Nahmani M. Gore A, et al. Front Synaptic Neurosci. 2022 Aug 11;14:968404. doi: 10.3389/fnsyn.2022.968404. eCollection 2022. Front Synaptic Neurosci. 2022. PMID: 36032419 Free PMC article. - Quantitative 3-D morphometric analysis of individual dendritic spines.
Basu S, Saha PK, Roszkowska M, Magnowska M, Baczynska E, Das N, Plewczynski D, Wlodarczyk J. Basu S, et al. Sci Rep. 2018 Feb 23;8(1):3545. doi: 10.1038/s41598-018-21753-8. Sci Rep. 2018. PMID: 29476060 Free PMC article. - Lipid dynamics at dendritic spines.
Dotti CG, Esteban JA, Ledesma MD. Dotti CG, et al. Front Neuroanat. 2014 Aug 8;8:76. doi: 10.3389/fnana.2014.00076. eCollection 2014. Front Neuroanat. 2014. PMID: 25152717 Free PMC article. Review. - The role of phosphoinositides in synapse function.
Ueda Y. Ueda Y. Mol Neurobiol. 2014 Dec;50(3):821-38. doi: 10.1007/s12035-014-8768-8. Epub 2014 Jun 17. Mol Neurobiol. 2014. PMID: 24935718 Review. - Structure, Distribution, and Function of Neuronal/Synaptic Spinules and Related Invaginating Projections.
Petralia RS, Wang YX, Mattson MP, Yao PJ. Petralia RS, et al. Neuromolecular Med. 2015 Sep;17(3):211-40. doi: 10.1007/s12017-015-8358-6. Epub 2015 May 26. Neuromolecular Med. 2015. PMID: 26007200 Free PMC article. Review.
References
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002) Molecular biology of the cell, Ed 4, pp 742-757. New York: Garland.
- Altman J (1993) Postnatal development of the cerebellar cortex in the rat II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol 145: 399-464. - PubMed
- Andres K (1964) Mikropinozytose im zentralnervensystem. In: Zietschrift four zellforschung und mikroskopische anatomie (Zellforsch Z, ed), pp 63-73. Vienna: Springer. - PubMed
Publication types
MeSH terms
Grants and funding
- NS33574/NS/NINDS NIH HHS/United States
- R01 EB002170/EB/NIBIB NIH HHS/United States
- NS21184/NS/NINDS NIH HHS/United States
- EB002170/EB/NIBIB NIH HHS/United States
- R01 NS021184/NS/NINDS NIH HHS/United States
- R37 NS021184/NS/NINDS NIH HHS/United States
- R01 NS033574/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous