Sequential activation of phosphatidylinositol 3-kinase, beta Pix, Rac1, and Nox1 in growth factor-induced production of H2O2 - PubMed (original) (raw)
Sequential activation of phosphatidylinositol 3-kinase, beta Pix, Rac1, and Nox1 in growth factor-induced production of H2O2
Hye Sun Park et al. Mol Cell Biol. 2004 May.
Abstract
The generation of reactive oxygen species (ROS) in cells stimulated with growth factors requires the activation of phosphatidylinositol 3-kinase (PI3K) and the Rac protein. We report here that the COOH-terminal region of Nox1, a protein related to gp91(phox) (Nox2) of phagocytic cells, is constitutively associated with beta Pix, a guanine nucleotide exchange factor for Rac. Both growth factor-induced ROS production and Rac1 activation were completely blocked in cells depleted of beta Pix by RNA interference. Rac1 was also shown to bind to the COOH-terminal region of Nox1 in a growth factor-dependent manner. Moreover, the depletion of Nox1 by RNA interference inhibited growth factor-induced ROS generation. These results suggest that ROS production in growth factor-stimulated cells is mediated by the sequential activation of PI3K, beta Pix, and Rac1, which then binds to Nox1 to stimulate its NADPH oxidase activity.
Figures
FIG. 1.
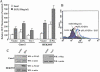
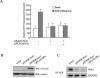
Effect of βPix overexpression on EGF-induced ROS generation in Caco-2 or HEK293T cells. (A) Caco-2 or HEK293T cells were transfected with an empty vector (pFLAG-CMV) or a vector encoding FLAG-tagged βPix or Myc-tagged Vav1. After incubation with EGF for 10 min, the generation of H2O2 was monitored by confocal microscopic analysis of DCF fluorescence (see Materials and Methods). The mean fluorescence intensity of DCF in experiments was measured and expressed relative to that of unstimulated cells transfected with empty vector. Data are means ± standard errors (SE) of values from five independent experiments. (B) HEK293T cells were transfected with empty vector (pFLAG-CMV) or with a vector encoding FLAG-tagged βPix. Cells were stimulated with EGF for 10 min and then stained with DCF-DA. The fluorescence was analyzed in 10,000 cells each with FACSCalibur (Becton Dickinson), with excitation at 488 nm and emission at 530 nm. (C) Cell lysates from each sample from panels A and B were prepared and subjected to immunoblot analysis (WB) with antibodies to FLAG (for recombinant βPix) or Myc (for recombinant Vav1); the filter was reprobed with antibodies to β-actin.
FIG. 2.
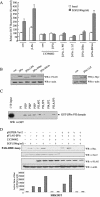
Effect of βPix depletion by siRNA expression on growth factor-induced ROS generation. (A) Two different cell lines (Caco-2 cells and HEK293T cells) were transfected with either pSUPER-βPix, encoding a βPix-specific siRNA, or pSUPER-Vav2, encoding a Vav2-specific siRNA. Control cells were transfected with only the vector control (pSUPER). After being cultured for 48 h, the cells were deprived of serum for 12 h. After EGF treatment for 10 min, the generation of H2O2 was then monitored by confocal microscopic analysis of DCF fluorescence. Data are means ± SE of values from five independent experiments. (B) Cell lysates were then prepared and subjected to immunoblot analysis with antibodies to βPix and Vav2 (Babraham Co., Cambridge, United Kingdom); the filter was reprobed with antibodies to β-actin. (C) HEK293T cells were transfected with pSUPER (control) or pSUPER-βPix. After being cultured for 48 h, the cells were deprived of serum for 12 h. After EGF treatment for 10 min, cell lysates were then prepared with PBS containing 1% Triton X-100 and were incubated for 3 h with a GST fusion protein of PAK-RBD conjugated to glutathione-Sepharose 4B beads. The beads were then washed with ice-cold cell lysis buffer, and bound proteins were subjected to immunoblot analysis with antibodies to Rac1 (upper panel). Cell lysates were also directly subjected to immunoblot analysis with antibodies to Rac (second panel), βPix (third panel), and β-actin (bottom panel).
FIG.3.
Roles of the PH domain and LZ motif of βPix in EGF-induced ROS generation. (A) Caco-2 cells were transfected with empty vector (pFLAG-CMV, or pcDNA3.0 for Myc epitope-tagged βPix-ΔLZ) or with vectors encoding either wild-type βPix or βPix mutants. They were then deprived of serum for 16 h and incubated for 10 min in the absence or presence of EGF (100 ng/ml). Cells were pretreated with LY294002 (10 μM) for 30 min before the treatment with EGF (100 ng/ml). The generation of H2O2 was assayed on the basis of DCF fluorescence. Data are means ± SE of values from five independent experiments. (B) Lysates of the transfected cells were subjected to immunoblot analysis with antibodies to the FLAG or Myc epitope, as indicated. (C) Phosphoinositide binding assay. Purified GST-βPix-PH domain (∼200 ng) was incubated with phosphoinositide analogue beads (Echelon Research Laboratories Inc.) overnight at 4°C and then washed with PBS containing 1% Triton X-100. Bead-bound proteins were separated by SDS-PAGE and analyzed by immunoblotting with antibodies against GST. (D) HEK293T cells were transfected with FLAG-βPix only or together with pSUPER-Vav2. After being cultured for 48 h, the cells were deprived of serum for 12 h. The cells were pretreated with LY294002 (10 μM) for 30 min before a treatment with EGF (100 ng/ml). After EGF treatment for 10 min, cell lysates were then prepared in PBS containing 1% Triton X-100 and were incubated for 3 h with a GST fusion protein of PAK-RBD conjugated to glutathione-Sepharose 4B beads. The beads were then washed with ice-cold cell lysis buffer, and bound proteins were subjected to immunoblot analysis with antibodies to Rac1 (top). Cell lysates were also directly subjected to immunoblot analysis with antibodies to Vav2 (Babraham Co.), FLAG, and β-actin. The quantitative results for an active form of Rac from the PAK-RBD assay are shown in graph form (bottom).
FIG. 4.
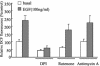
Effect of pharmacological inhibitors on EGF-induced ROS generation. Caco-2 cells were pretreated with DPI (10 μM), rotenone (1 μM), and antimycin A (25 mg/ml) for 30 min before treatment with EGF. After EGF (100 ng/ml) treatment for 10 min, the generation of H2O2 was measured on the basis of DCF fluorescence. Data are means ± SE of values from three independent experiments.
FIG. 5.
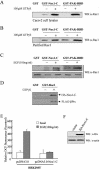
Growth factor-independent interaction of βPix with Nox1. (A) Schematic representation of the structure of Nox1. The horizontal line represents Nox1-C. (B) Caco-2 cells were transfected with FLAG-tagged βPix. After serum deprivation, they were incubated in the absence or presence of EGF (100 ng/ml) for 10 min. Cell lysates were then prepared, incubated for 2 h with bead-conjugated GST or GST-Nox1-C, and then subjected to immunoblot analysis with antibodies to FLAG. (C) pFLAG-CMV-βPix was transiently expressed in HEK293T cells either alone or together with pcDNA3.0-HA-Nox1-C, as indicated. After serum starvation for 16 h, cells were incubated in the absence or presence of EGF (100 ng/ml) for 10 min. Cell lysates were then subjected to immunoprecipitation (IP) with antibodies to HA, and the resulting precipitates were subjected to immunoblot analysis with antibodies to FLAG (upper panel). Lysates were also directly subjected to immunoblot analysis with antibodies to HA (middle panel) or FLAG (lower panel). (D) Cos-7 cells cotransfected with pEGFP-N1-Nox1 and pFLAG-βPix were starved of serum for 16 h and then fixed before (top panels) or after (bottom panels) stimulation with EGF (100 ng/ml) for 10 min. βPix distribution was visualized by using an anti-FLAG antibody and a tetramethyl rhodamine isocyanate-conjugated secondary antibody. These results are representative of three independent experiments.
FIG. 5.
Growth factor-independent interaction of βPix with Nox1. (A) Schematic representation of the structure of Nox1. The horizontal line represents Nox1-C. (B) Caco-2 cells were transfected with FLAG-tagged βPix. After serum deprivation, they were incubated in the absence or presence of EGF (100 ng/ml) for 10 min. Cell lysates were then prepared, incubated for 2 h with bead-conjugated GST or GST-Nox1-C, and then subjected to immunoblot analysis with antibodies to FLAG. (C) pFLAG-CMV-βPix was transiently expressed in HEK293T cells either alone or together with pcDNA3.0-HA-Nox1-C, as indicated. After serum starvation for 16 h, cells were incubated in the absence or presence of EGF (100 ng/ml) for 10 min. Cell lysates were then subjected to immunoprecipitation (IP) with antibodies to HA, and the resulting precipitates were subjected to immunoblot analysis with antibodies to FLAG (upper panel). Lysates were also directly subjected to immunoblot analysis with antibodies to HA (middle panel) or FLAG (lower panel). (D) Cos-7 cells cotransfected with pEGFP-N1-Nox1 and pFLAG-βPix were starved of serum for 16 h and then fixed before (top panels) or after (bottom panels) stimulation with EGF (100 ng/ml) for 10 min. βPix distribution was visualized by using an anti-FLAG antibody and a tetramethyl rhodamine isocyanate-conjugated secondary antibody. These results are representative of three independent experiments.
FIG. 6.
Direct interaction of Rac1 with Nox1. A Caco-2 cell lysate (A) or purified Rac1 (1 μg) (B) was incubated first for 10 min at room temperature in the absence or presence of 100 μM GTP-γ-S and then for 3 h at 4°C with bead-conjugated GST, GST-Nox1-C, or GST-PAK-RBD (10 μg) in a final volume of 500 μl of PBS containing 1% Triton X-100 in the continued absence or presence of 100 μM GTP-γ-S. Proteins that bound specifically to the beads as well as to the cell lysate or purified Rac1 were then subjected to immunoblot analysis with antibodies to Rac1. (C) Caco-2 cells were deprived of serum for 16 h and then incubated for 10 min in the absence or presence of EGF (100 ng/ml). Cell lysates were then incubated for 3 h with bead-bound GST, GST-Nox1-C, or GST-PAK-RBD. Proteins retained specifically by the beads were subjected to immunoblot analysis with antibodies to Rac1 (upper panel). Cell lysates were also subjected directly to immunoblot analysis with the same antibodies (lower panel). (D) GST-Rac was incubated first for 10 min at room temperature in the absence or presence of 100 μM GTP-γ-S and then with lysates of HEK293T cells transfected with HA-Nox1-C together with FLAG-βPix for 3 h at 4°C. The beads were then washed with ice-cold cell lysis buffer, and bound proteins were subjected to immunoblot analysis with antibodies to HA or FLAG. (E) HEK293T cells transfected with a vector encoding Nox1-C (pcDNA3.0-HA-Nox1-C) or the corresponding empty vector (pcDNA3.0) were deprived of serum for 16 h and then incubated for 10 min in the absence or presence of EGF (100 ng/ml). The generation of H2O2 was assayed on the basis of DCF fluorescence. Data are expressed as mean fluorescence intensities relative to that for the corresponding unstimulated cells transfected with empty vector and are means ± SE of values from five independent experiments. (F) Cells transfected as for panel E were lysed and subjected to immunoblot analysis with antibodies to HA; filters were reprobed with antibodies to β-actin.
FIG. 7.
Role of Nox1 in EGF-induced ROS generation. (A) Caco-2 cells were electroporated with either pFLAG-βPix or the empty vector together with pSUPER-Nox1. After being cultured for 48 h, the cells were deprived of serum for 12 h. After EGF (100 ng/ml) treatment, the generation of H2O2 was measured on the basis of DCF fluorescence. Data are means ± SE of values from three independent experiments. (B) Total cell lysates of each sample from panel A were prepared and subjected to immunoblot analysis with antibodies to FLAG; the filter was then reprobed with antibodies to β-actin. (C) Total RNA was prepared from each sample from panel A. An RT-PCR demonstrates Nox1 expression. GAPDH served as a loading control.
Similar articles
- Nox1-dependent reactive oxygen generation is regulated by Rac1.
Cheng G, Diebold BA, Hughes Y, Lambeth JD. Cheng G, et al. J Biol Chem. 2006 Jun 30;281(26):17718-26. doi: 10.1074/jbc.M512751200. Epub 2006 Apr 24. J Biol Chem. 2006. PMID: 16636067 - Phosphatidylinositol 3-kinase-dependent membrane recruitment of Rac-1 and p47phox is critical for alpha-platelet-derived growth factor receptor-induced production of reactive oxygen species.
Bäumer AT, Ten Freyhaus H, Sauer H, Wartenberg M, Kappert K, Schnabel P, Konkol C, Hescheler J, Vantler M, Rosenkranz S. Bäumer AT, et al. J Biol Chem. 2008 Mar 21;283(12):7864-76. doi: 10.1074/jbc.M704997200. Epub 2007 Dec 10. J Biol Chem. 2008. PMID: 18070887 - Targeting and activation of Rac1 are mediated by the exchange factor beta-Pix.
ten Klooster JP, Jaffer ZM, Chernoff J, Hordijk PL. ten Klooster JP, et al. J Cell Biol. 2006 Feb 27;172(5):759-69. doi: 10.1083/jcb.200509096. Epub 2006 Feb 21. J Cell Biol. 2006. PMID: 16492808 Free PMC article. - Protein kinase A-dependent phosphorylation modulates beta1Pix guanine nucleotide exchange factor activity through 14-3-3beta binding.
Chahdi A, Sorokin A. Chahdi A, et al. Mol Cell Biol. 2008 Mar;28(5):1679-87. doi: 10.1128/MCB.00898-07. Epub 2007 Dec 26. Mol Cell Biol. 2008. PMID: 18160719 Free PMC article. - Alsin and SOD1(G93A) proteins regulate endosomal reactive oxygen species production by glial cells and proinflammatory pathways responsible for neurotoxicity.
Li Q, Spencer NY, Pantazis NJ, Engelhardt JF. Li Q, et al. J Biol Chem. 2011 Nov 18;286(46):40151-62. doi: 10.1074/jbc.M111.279711. Epub 2011 Sep 20. J Biol Chem. 2011. PMID: 21937428 Free PMC article.
Cited by
- Copper influx transporter 1 is required for FGF, PDGF and EGF-induced MAPK signaling.
Tsai CY, Finley JC, Ali SS, Patel HH, Howell SB. Tsai CY, et al. Biochem Pharmacol. 2012 Oct 15;84(8):1007-13. doi: 10.1016/j.bcp.2012.07.014. Epub 2012 Jul 25. Biochem Pharmacol. 2012. PMID: 22842628 Free PMC article. - Phosphorylation of the cool-1/beta-Pix protein serves as a regulatory signal for the migration and invasive activity of Src-transformed cells.
Feng Q, Baird D, Yoo S, Antonyak M, Cerione RA. Feng Q, et al. J Biol Chem. 2010 Jun 11;285(24):18806-16. doi: 10.1074/jbc.M109.098079. Epub 2010 Apr 7. J Biol Chem. 2010. PMID: 20375009 Free PMC article. - 7-Dehydrocholesterol enhances ultraviolet A-induced oxidative stress in keratinocytes: roles of NADPH oxidase, mitochondria, and lipid rafts.
Valencia A, Rajadurai A, Carle AB, Kochevar IE. Valencia A, et al. Free Radic Biol Med. 2006 Dec 1;41(11):1704-18. doi: 10.1016/j.freeradbiomed.2006.09.006. Epub 2006 Sep 9. Free Radic Biol Med. 2006. PMID: 17145559 Free PMC article. - Reactive oxygen species signaling in vascular smooth muscle cells.
Clempus RE, Griendling KK. Clempus RE, et al. Cardiovasc Res. 2006 Jul 15;71(2):216-25. doi: 10.1016/j.cardiores.2006.02.033. Epub 2006 Mar 7. Cardiovasc Res. 2006. PMID: 16616906 Free PMC article. Review. - Lipid rafts and caveolin-1 coordinate interleukin-1beta (IL-1beta)-dependent activation of NFkappaB by controlling endocytosis of Nox2 and IL-1beta receptor 1 from the plasma membrane.
Oakley FD, Smith RL, Engelhardt JF. Oakley FD, et al. J Biol Chem. 2009 Nov 27;284(48):33255-64. doi: 10.1074/jbc.M109.042127. Epub 2009 Oct 1. J Biol Chem. 2009. PMID: 19801678 Free PMC article.
References
- Ago, T., H. Nunoi, T. Ito, and H. Sumimoto. 1999. Mechanism for phosphorylation-induced activation of the phagocyte NADPH oxidase protein p47phox. J. Biol. Chem. 274:33644-33653. - PubMed
- Babior, B. M. 1999. NADPH oxidase. Blood 93:1464-1476. - PubMed
- Bae, Y. S., S. W. Kang, M. S. Seo, I. C. Baines, E. Tekle, P. B. Chock, and S. G. Rhee. 1997. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 272:217-221. - PubMed
- Bae, Y. S., J. Y. Sung, O. S. Kim, Y. J. Kim, K. C. Hur, A. Kazlauskas, and S. G. Rhee. 2000. Platelet-derived growth factor-induced H2O2 production requires the activation of phosphatidylinositol 3-kinase. J. Biol. Chem. 275:10527-10531. - PubMed
- Bokoch, G. M., and U. G. Knaus. 2003. NADPH oxidases: not just for leukocytes anymore. Trends Biochem. Sci. 9:502-508. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous