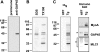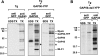Identification of the membrane receptor of a class XIV myosin in Toxoplasma gondii - PubMed (original) (raw)
Identification of the membrane receptor of a class XIV myosin in Toxoplasma gondii
Elizabeth Gaskins et al. J Cell Biol. 2004.
Abstract
Apicomplexan parasites exhibit a unique form of substrate-dependent motility, gliding motility, which is essential during their invasion of host cells and during their spread between host cells. This process is dependent on actin filaments and myosin that are both located between the plasma membrane and two underlying membranes of the inner membrane complex. We have identified a protein complex in the apicomplexan parasite Toxoplasma gondii that contains the class XIV myosin required for gliding motility, TgMyoA, its associated light chain, TgMLC1, and two novel proteins, TgGAP45 and TgGAP50. We have localized this complex to the inner membrane complex of Toxoplasma, where it is anchored in the membrane by TgGAP50, an integral membrane glycoprotein. Assembly of the protein complex is spatially controlled and occurs in two stages. These results provide the first molecular description of an integral membrane protein as a specific receptor for a myosin motor, and further our understanding of the motile apparatus underlying gliding motility in apicomplexan parasites.
Copyright the Rockefeller University Press
Figures
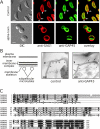
Figure 1.
GAP45 is associated with the inner membrane complex of T. gondii. (A) Distribution of TgGAP45 was compared with that of the plasma membrane protein SAG1 in control cells and in parasites treated with C. septicum α-toxin. In untreated cells, the plasma membrane protein SAG1 is evenly distributed over the parasite circumference, whereas TgGAP45 distribution reveals an anterior gap (arrows) consistent with localization to the inner membrane complex. In toxin-treated parasites, the plasma membrane is markedly distended, whereas the distribution of TgGAP45 is not affected, confirming its association with the inner membrane complex. Bars, 4 μm. (B) Diagram illustrates the basic elements of the Toxoplasma pellicle: the plasma membrane, the two membranes of the inner membrane complex, the membrane skeleton, and the subpellicular microtubules. Isolated pellicle preparations were incubated with control antiserum or TgGAP45 antiserum, followed by goat anti–rabbit secondary antibodies conjugated to 10-nm gold. The arrowhead indicates the plasma membrane, the arrows the gold particles, and the asterisks the subpellicular microtubules. Bars, 150 nm. (C) Multiple alignment of GAP45 sequences from different apicomplexan parasites. Amino acid residues identical in at least three of the four sequences are highlighted in black; similar residues in gray. The amino-terminal N-myristoylation motif in the GAP45 sequences is indicated with an asterisk. TgGAP45: T. gondii GAP45, GenBank/EBML/DDBJ accession no. AAP41369; NcGAP45: N. caninum GAP45 sequence, assembled from EST sequences with accession no. NcEST3c79 and NcEST3d11b08; PfGAP45: P. falciparum GAP45, accession no. AAN36304; PyGAP45: P. yoelii GAP45, accession no. EAA23022; CpGAP45: C. parvum GAP45, accession no. CAD98387.
Figure 2.
GAP45 is associated with myosin-A, myosin light chain-1, and a novel 50-kD protein. (A) The TgGAP45 antiserum recognizes a single 45-kD protein on immunoblots. A Toxoplasma lysate was fractionated by SDS-PAGE and immunoblot analysis using either preimmune serum or a TgGAP45 antiserum. (B) Immunoprecipitation analysis with the TgGAP45 antiserum. Intracellular parasites were metabolically labeled with [35S]-labeled methionine and cysteine, lysed in a buffer containing either SDS or TX100, and subjected to immunoprecipitation with the TgGAP45 antiserum. Only one protein, GAP45, is immunoprecipitated from denaturing extracts prepared with SDS, whereas three additional proteins are immunoprecipitated from TX100 extracts. (C) Metabolically labeled parasites or HFF cells were lysed in TX100-containing buffer and subjected to immunoprecipitation with the TgGAP45 antiserum. The immune complex was subjected to SDS-PAGE and transferred to nitrocellulose. Different molecular mass ranges of the blot were excised and incubated with monospecific antisera to TgMyoA, TgGAP45, and TgMLC1; each recognize their respective proteins in the immunoprecipitated complex. Asterisks indicate the IgG heavy and light chains that cross react with the secondary antibody used.
Figure 3.
Multiple alignment of Toxoplasma GAP50 with its apicomplexan orthologues and rat purple acid phosphatase. The predicted amino acid sequence of TgGAP50 was aligned with its orthologues in P. falciparum, P. yoelii, and E. tenella and with rat purple acid phosphatase using ClustalW. Amino acid residues identical in at least three of the four sequences are highlighted in black; similar residues in gray. Arrow indicates the amino terminus of mature TgGAP50 as determined by direct protein sequencing. Lines indicate the sequences of tryptic peptides of TgGAP50 as determined by mass spectroscopy. The putative carboxy-terminal transmembrane domain of TgGAP50 is boxed. Potential N-linked glycosylation sites in TgGAP50 and EtGAP50 and actual sites in the rat phosphatase are indicated by “###.” The amino acid residues in rat APP5 required for metal binding and enzymatic activity are labeled with asterisks. TgGAP50: T. gondii GAP50, GenBank/EBML/DDBJ accession no. AY587763; EtGAP50: E. tenella GAP50, this sequence was generated from unassembled shotgun reads of the E. tenella genome available at
http://www.sanger.ac.uk/Projects/E\_tenella/
; PfGAP50: P. falciparum GAP50, accession no. NP_704719; PyGAP50: P. yoelii GAP50, accession no. EAA16957; RnAPP5: rat tartrate-resistant acid phosphatase type 5 precursor, accession no. P29288.
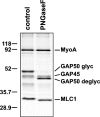
Figure 4.
Toxoplasma GAP50 is modified by N-linked glycosylation. The glideosome complex was immunoprecipitated from metabolically labeled parasites and was incubated in either the absence or presence of PNGase-F. Bands corresponding to the fully glycosylated and deglycosylated TgGAP50 are indicated.
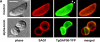
Figure 5.
GAP50 is an inner membrane complex protein and the glideosome is assembled in two stages. (A) Distribution of TgGAP50-YFP was compared with that of the plasma membrane protein SAG1 in control cells and in parasites treated with C. septicum α-toxin. In untreated cells, the plasma membrane protein SAG1 is evenly distributed over the parasite circumference, whereas an anterior gap in TgGAP50-YFP distribution (arrows) is consistent with localization to the inner membrane complex. In toxin-treated parasites, the plasma membrane is markedly distended, whereas the distribution of TgGAP50-YFP is not affected, confirming its association with the inner membrane complex. Bars, 4 μm. (B) Localization of glideosome components TgGAP50, TgGAP45, TgMyoA, and TgMLC1 relative to each other and the Toxoplasma plasma membrane and inner membrane complex was determined by immunofluorescence analysis of Toxoplasma expressing a TgGAP50-YFP fusion protein and HA-tagged TgMyoA. TgGAP45 and TgMLC1 were detected using monospecific antisera and HA-TgMyoA using an anti-HA epitope mAb. TgGAP50 is found in the inner membrane complexes of both the mature parasite and immature daughter parasites. In contrast, TgMyoA, TgMLC1, and TgGAP45 are only found associated with the inner membrane complex of the mature parasite. Bars, 4 μm. The diagram shows the localization of the plasma membrane of a mother parasite and of the inner membrane complexes of mother and daughter parasites. The inner membrane complex was detected using monospecific antisera to TgIMC1.
Figure 5.
GAP50 is an inner membrane complex protein and the glideosome is assembled in two stages. (A) Distribution of TgGAP50-YFP was compared with that of the plasma membrane protein SAG1 in control cells and in parasites treated with C. septicum α-toxin. In untreated cells, the plasma membrane protein SAG1 is evenly distributed over the parasite circumference, whereas an anterior gap in TgGAP50-YFP distribution (arrows) is consistent with localization to the inner membrane complex. In toxin-treated parasites, the plasma membrane is markedly distended, whereas the distribution of TgGAP50-YFP is not affected, confirming its association with the inner membrane complex. Bars, 4 μm. (B) Localization of glideosome components TgGAP50, TgGAP45, TgMyoA, and TgMLC1 relative to each other and the Toxoplasma plasma membrane and inner membrane complex was determined by immunofluorescence analysis of Toxoplasma expressing a TgGAP50-YFP fusion protein and HA-tagged TgMyoA. TgGAP45 and TgMLC1 were detected using monospecific antisera and HA-TgMyoA using an anti-HA epitope mAb. TgGAP50 is found in the inner membrane complexes of both the mature parasite and immature daughter parasites. In contrast, TgMyoA, TgMLC1, and TgGAP45 are only found associated with the inner membrane complex of the mature parasite. Bars, 4 μm. The diagram shows the localization of the plasma membrane of a mother parasite and of the inner membrane complexes of mother and daughter parasites. The inner membrane complex was detected using monospecific antisera to TgIMC1.
Figure 6.
The carboxy-terminal cytoplasmic tail of TgGAP50 interacts with the other members of the glideosome complex. (A) Toxoplasma expressing a TgGAP50-YFP fusion protein and nontransfected parasites were metabolically labeled with [35S]-labeled methionine and cysteine and after lysis in either SDS or TX100 lysis buffer subjected to immunoprecipitation with antiserum to TgGAP45 or GFP, which cross reacts with YFP. The other three members of the complex are coimmunoprecipitated with TgGAP50-YFP. (B) Toxoplasma transiently expressing a TgGAP50Δ(427-431)YFP fusion protein were metabolically labeled with [35S]-labeled methionine and cysteine, and after lysis in TX100 lysis buffer were subjected to immunoprecipitation with antisera to either TgGAP45 or GFP. The prominent 40-kD band (A, asterisk) is a breakdown product of TgGAP45. Unlike full-length TgGAP50-YFP, TgGAP50Δtail-YFP does not interact with the glideosome complex.
Figure 7.
The glideosome complex is assembled in two stages and the presence of GAP50 correlates with membrane association. (A) Parallel cultures of _Toxoplasma_-infected HFF cells were metabolically labeled for 15 min at 37°C with [35S]-labeled methionine and cysteine, and were either placed on ice (pulse) or incubated for an additional 4 h at 37°C after addition of unlabeled amino acids (chase). Parasites were isolated, lysed in TX100 lysis buffer, and subjected to immunoprecipitation with TgGAP45 antiserum. (B) Parallel cultures of _Toxoplasma_-infected HFF cells were pulse labeled and chased as described above. Parasites were homogenized by sonication and an aliquot was fractionated into soluble (SN) and particulate (P) fractions by centrifugation. These were solubilized in TX100 lysis buffer along with the nonfractionated sample and subjected to immunoprecipitation with the TgGAP45 antiserum. We routinely observed that TgGAP45 in the pulsed sample migrated as a doublet, irrespective of whether it was solubilized in TX100 or SDS.
Similar articles
- Blocking Palmitoylation of Toxoplasma gondii Myosin Light Chain 1 Disrupts Glideosome Composition but Has Little Impact on Parasite Motility.
Rompikuntal PK, Kent RS, Foe IT, Deng B, Bogyo M, Ward GE. Rompikuntal PK, et al. mSphere. 2021 May 19;6(3):e00823-20. doi: 10.1128/mSphere.00823-20. mSphere. 2021. PMID: 34011689 Free PMC article. - A small-molecule inhibitor of T. gondii motility induces the posttranslational modification of myosin light chain-1 and inhibits myosin motor activity.
Heaslip AT, Leung JM, Carey KL, Catti F, Warshaw DM, Westwood NJ, Ballif BA, Ward GE. Heaslip AT, et al. PLoS Pathog. 2010 Jan 15;6(1):e1000720. doi: 10.1371/journal.ppat.1000720. PLoS Pathog. 2010. PMID: 20084115 Free PMC article. - Myosin A tail domain interacting protein (MTIP) localizes to the inner membrane complex of Plasmodium sporozoites.
Bergman LW, Kaiser K, Fujioka H, Coppens I, Daly TM, Fox S, Matuschewski K, Nussenzweig V, Kappe SH. Bergman LW, et al. J Cell Sci. 2003 Jan 1;116(Pt 1):39-49. doi: 10.1242/jcs.00194. J Cell Sci. 2003. PMID: 12456714 - Gliding motility in apicomplexan parasites.
Heintzelman MB. Heintzelman MB. Semin Cell Dev Biol. 2015 Oct;46:135-42. doi: 10.1016/j.semcdb.2015.09.020. Epub 2015 Sep 30. Semin Cell Dev Biol. 2015. PMID: 26428297 Review. - Environmental sensing and regulation of motility in Toxoplasma.
Uboldi AD, Wilde ML, Bader SM, Tonkin CJ. Uboldi AD, et al. Mol Microbiol. 2021 May;115(5):916-929. doi: 10.1111/mmi.14661. Epub 2020 Dec 28. Mol Microbiol. 2021. PMID: 33278047 Review.
Cited by
- The Modular Circuitry of Apicomplexan Cell Division Plasticity.
Gubbels MJ, Coppens I, Zarringhalam K, Duraisingh MT, Engelberg K. Gubbels MJ, et al. Front Cell Infect Microbiol. 2021 Apr 12;11:670049. doi: 10.3389/fcimb.2021.670049. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33912479 Free PMC article. Review. - Cytokinetic abscission in Toxoplasma gondii is governed by protein phosphatase 2A and the daughter cell scaffold complex.
Marq JB, Gosetto M, Altenried A, Vadas O, Maco B, Dos Santos Pacheco N, Tosetti N, Soldati-Favre D, Lentini G. Marq JB, et al. EMBO J. 2024 Sep;43(17):3752-3786. doi: 10.1038/s44318-024-00171-9. Epub 2024 Jul 15. EMBO J. 2024. PMID: 39009675 Free PMC article. - Plasmodium berghei calcium-dependent protein kinase 3 is required for ookinete gliding motility and mosquito midgut invasion.
Siden-Kiamos I, Ecker A, Nybäck S, Louis C, Sinden RE, Billker O. Siden-Kiamos I, et al. Mol Microbiol. 2006 Jun;60(6):1355-63. doi: 10.1111/j.1365-2958.2006.05189.x. Mol Microbiol. 2006. PMID: 16796674 Free PMC article. - A family of intermediate filament-like proteins is sequentially assembled into the cytoskeleton of Toxoplasma gondii.
Anderson-White BR, Ivey FD, Cheng K, Szatanek T, Lorestani A, Beckers CJ, Ferguson DJ, Sahoo N, Gubbels MJ. Anderson-White BR, et al. Cell Microbiol. 2011 Jan;13(1):18-31. doi: 10.1111/j.1462-5822.2010.01514.x. Cell Microbiol. 2011. PMID: 20698859 Free PMC article. - A highly dynamic F-actin network regulates transport and recycling of micronemes in Toxoplasma gondii vacuoles.
Periz J, Del Rosario M, McStea A, Gras S, Loney C, Wang L, Martin-Fernandez ML, Meissner M. Periz J, et al. Nat Commun. 2019 Sep 13;10(1):4183. doi: 10.1038/s41467-019-12136-2. Nat Commun. 2019. PMID: 31519913 Free PMC article.
References
- Bergman, L.W., K. Kaiser, H. Fujioka, I. Coppens, T.M. Daly, S. Fox, K. Matuschewski, V. Nussenzweig, and S.H. Kappe. 2003. Myosin A tail domain interacting protein (MTIP) localizes to the inner membrane complex of Plasmodium sporozoites. J. Cell Sci. 116:39–49. - PubMed
- Dobrowolski, J.M., and L.D. Sibley. 1996. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell. 84:933–939. - PubMed
- Dobrowolski, J.M., V.B. Carruthers, and L.D. Sibley. 1997. Participation of myosin in gliding motility and host cell invasion by Toxoplasma gondii. Mol. Microbiol. 26:163–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI01719/AI/NIAID NIH HHS/United States
- R29 AI041765/AI/NIAID NIH HHS/United States
- R01 AI041765/AI/NIAID NIH HHS/United States
- 1RO1AI045806-01A1/AI/NIAID NIH HHS/United States
- K02 AI001719/AI/NIAID NIH HHS/United States
- AI41765/AI/NIAID NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R01 AI045806/AI/NIAID NIH HHS/United States
- AI05093/AI/NIAID NIH HHS/United States
- Z01 AI005093/ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources