Glucocorticoids synergize with IL-1beta to induce TLR2 expression via MAP Kinase Phosphatase-1-dependent dual Inhibition of MAPK JNK and p38 in epithelial cells - PubMed (original) (raw)
Comparative Study
Glucocorticoids synergize with IL-1beta to induce TLR2 expression via MAP Kinase Phosphatase-1-dependent dual Inhibition of MAPK JNK and p38 in epithelial cells
Akihiro Sakai et al. BMC Mol Biol. 2004.
Abstract
Background: Despite the importance of glucocorticoids in suppressing immune and inflammatory responses, their role in enhancing host immune and defense response against invading bacteria is poorly understood. Toll-like receptor 2 (TLR2) has recently gained importance as one of the major host defense receptors. The increased expression of TLR2 in response to bacteria-induced cytokines has been thought to be crucial for the accelerated immune response and resensitization of epithelial cells to invading pathogens.
Results: We show that IL-1beta, a key proinflammatory cytokine, greatly up-regulates TLR2 expression in human epithelial cells via a positive IKKbeta-IkappaBalpha-dependent NF-kappaB pathway and negative MEKK1-MKK4/7-JNK1/2 and MKK3/6-p38 alpha/beta pathways. Glucocorticoids synergistically enhance IL-1beta-induced TLR2 expression via specific up-regulation of the MAP kinase phosphatase-1 that, in turn, leads to dephosphorylation and inactivation of both MAPK JNK and p38, the negative regulators for TLR2 induction.
Conclusion: These results indicate that glucocorticoids not only suppress immune and inflammatory response, but also enhance the expression of the host defense receptor, TLR2. Thus, our studies may bring new insights into the novel role of glucocorticoids in orchestrating and optimizing host immune and defense responses during bacterial infections and enhance our understanding of the signaling mechanisms underlying the glucocorticoid-mediated attenuation of MAPK.
Figures
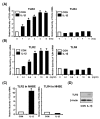
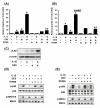
Figure 1
IL-1β up-regulates TLR2, but not TLR4, in human epithelial cells including primary bronchial epithelial cells. A, IL-1β up-regulates TLR2 mRNA, but not TLR4 mRNA, in HeLa cells in a time-dependent manner. Cells were treated with IL-1β (10 ng/ml) for the indicated time periods. The expression of TLR2 and TLR4 at mRNA level was then measured by real-time quantitative PCR in IL-1β-treated and -untreated human cervix epithelial HeLa cells. TLR2 and TLR4 mRNA levels were normalized to the level of cyclophilin that served as an internal control for the amount of RNA used in each reaction. Values are the mean ± SD; n = 3. The symbols (*) indicate results significantly different (p < 0.01) from unstimulated condition. B, IL-1β up-regulates TLR2 mRNA, but not TLR4 mRNA, in HeLa cells in a dose-dependent manner. HeLa cells were treated with the indicated concentration of IL-1β for 3 h. Values are the mean ± SD; n = 3. The symbols (*) indicate results significantly different (p < 0.01) from unstimulated condition. C, IL-1β up-regulates TLR2 mRNA, but not TLR4 mRNA, in primary human bronchial epithelial NHBE cells. NHBE cells were treated with IL-1β (10 ng/ml) for 3 h. Values are the mean ± SD; n = 3. The symbols (*) indicate results significantly different (p < 0.01) from unstimulated condition. D, IL-1β also induces expression of TLR2 at protein level in HeLa cells. HeLa cells were treated for 5 h with IL-1β (10 ng/ml). Protein level of TLR2 was then assessed by Western blot analysis using an anti-hTLR2 antibody. β-Actin was used as a protein loading control.
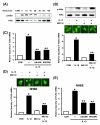
Figure 2
IKKβ-IκBα-dependent activation of NF-κB is required for IL-1β-induced TLR2 up-regulation. A, IL-1β induces IκBα phosphorylation and degradation in HeLa cells as assessed by Western blot analysis. Cells were treated with IL-1β (10 ng/ml) for the indicated time periods. B, MG 132 inhibits IL-1β-induced NF-κB nuclear translocation, IκBα degradation and TLR2 up-regulation in HeLa cells. For Western blotting and immunofluorescent staining, cells were pretreated with MG132 (1 μM) for 2 h prior to treatment with IL-1β (10 ng/ml) for 20 min. For the measurement of TLR2 mRNA, cells were treated with IL-1β (10 ng/ml) for 3 h after MG132 pretreatment. Values are the mean ± SD; n = 3. The symbols indicate results significantly different (p < 0.01) from unstimulated condition (*) and IL-1β-stimulated condition (without MG132)(**). C, Overexpression of a transdominant mutant of IκBα [IκBα [(S32A, S36A)] and a dominant-negative mutant of IKKβ [IKKβ (K44A)] inhibit IL-1β-induced TLR2 up-regulation in HeLa cells. HeLa cells were treated with IL-1β (10 ng/ml) for 3 h. Values are the mean ± SD; n = 3. The symbols indicate results significantly different (p < 0.01) from unstimulated condition (*) and IL-1β-stimulated condition (without dominant-negative construct)(**). D, MG 132 also inhibits IL-1β-induced NF-κB nuclear translocation and TLR2 up-regulation in NHBE cells. For immunofluorescent staining, cells were pretreated with MG132 (1 μM) for 2 h prior to treatment with IL-1β (10 ng/ml) for 20 min. For the measurement of TLR2 mRNA, cells were treated with IL-1β (10 ng/ml) for 3 h after MG132 pretreatment. Values are the mean ± SD; n = 3. The symbols indicate results significantly different (p < 0.01) from unstimulated condition (*) and IL-1β-stimulated condition (without MG132)(**). E, Overexpression of a transdominant mutant of IκBα (S32A, S36A) and a dominant-negative mutant of IKKβ (K44A) reduce IL-1β-induced TLR2 up-regulation in NHBE cells. Cells were treated with IL-1β (10 ng/ml) for 3 h. Values are the mean ± SD; n = 3. The symbols indicate results significantly different (p < 0.01) from unstimulated condition (*) and IL-1β-stimulated condition (without dominant-negative construct)(**).
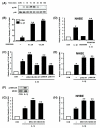
Figure 3
Activation of MKK3/6-p38 α/β MAPK pathway is negatively involved in IL-1β-induced TLR2 expression. A, IL-1β induces p38 phosphorylation in HeLa cells in a time-dependent manner as assessed by Western blot analysis. Cells were treated with IL-1β (10 ng/ml) for the indicated time periods. B, SB203580 greatly enhances IL-1β-induced TLR2 up-regulation in HeLa cells. Cells were pretreated for 2 h with the indicated concentration of SB203580 prior to treatment with IL-1β (10 ng/ml) for 3 h. Values are the mean ± SD; n = 3. The symbols indicate results significantly different (p < 0.01) from unstimulated condition (*) and IL-1β-stimulated condition (without SB203580)(**). C, Overexpression of a dominant-negative mutants of either p38α [fp38α(AF)] or p38β [fp38β (AF)] enhances, whereas overexpression of a wild-type p38α or p38β reduces, the IL-1β-induced TLR2 expression in HeLa cells. Cells were treated with IL-1β (10 ng/ml) for 3 h. Values are the mean ± SD; n = 3. The symbols indicate results significantly different (p < 0.01) from unstimulated condition (*) and IL-1β-stimulated condition (without wild-type or dominant-negative construct)(**). D, SB203580 greatly enhances IL-1β-induced TLR2 up-regulation in NHBE cells. Cells were pretreated for 2 h with SB203580 (20 μM) prior to treatment with IL-1β (10 ng/ml) for 3 h. Values are the mean ± SD; n = 3. The symbols indicate results significantly different (p < 0.01) from unstimulated condition (*) and IL-1β-stimulated condition (without SB203580)(**). E, Overexpression of a dominant-negative mutant of p38α or p38β enhances the IL-1β-induced TLR2 expression in NHBE cells. Values are the mean ± SD; n = 3. The symbols indicate results significantly different (p < 0.01) from unstimulated condition (*) and IL-1β-stimulated condition (without dominant negative construct)(**). F, IL-1β induces MKK3/6 phosphorylation in HeLa cells. Cells were treated with IL-1β (10 ng/ml) for 20 min. G, Overexpression of a dominant-negative mutant form of either MKK3 [MKK3b (A)] or MKK6 [MKK6b (A)] also enhances IL-1β-induced TLR2 up-regulation in HeLa cells. Cells were treated with IL-1β (10 ng/ml) for 3 h. Values are the mean ± SD; n = 3. The symbols indicate results significantly different (p < 0.01) from unstimulated condition (*) and IL-1β-stimulated condition (without dominant-negative construct)(**). H, Overexpression of a dominant-negative mutant form of either MKK3 or MKK6 also enhances IL-1β-induced TLR2 up-regulation in NHBE cells. Cells were treated with IL-1β (10 ng/ml) for 3 h. Values are the mean ± SD; n = 3. The symbols indicate results significantly different (p < 0.01) from unstimulated condition (*) and IL-1β-stimulated condition (without dominant-negative construct)(**).
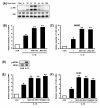
Figure 4
Activation of MEKK1-MKK4/7-JNK1/2 pathway is also negatively involved in IL-1β-induced TLR2 expression. A, IL-1β induces JNK phosphorylation in HeLa cells in a time-dependent manner as assessed by Western blot analysis. Cells were treated with IL-1β (10 ng/ml) for the indicated time periods. B, Overexpression of a dominant-negative mutants of either JNK1 [JNK1 (AF)] or JNK2 [JNK2 (AF)] enhances the IL-1β-induced TLR2 expressionin HeLa cells. Values are the mean ± SD; n = 3. The symbols indicate results significantly different (p < 0.01) from unstimulated condition (*) and IL-1β-stimulated condition (without dominant-negative construct)(**). C, Overexpression of a dominant-negative mutant of either JNK1 or JNK2 enhances the IL-1β-induced TLR2 expression in NHBE cells. Values are the mean ± SD; n = 3. The symbols indicate results significantly different (p < 0.01) from unstimulated condition (*) and IL-1β-stimulated condition (without dominant-negative construct)(**). D, IL-1β induces MKK4 phosphorylation in HeLa cells. Cells were treated with IL-1β (10 ng/ml) for 20 min. E, Overexpression of a dominant-negative mutant form of MKK4 or MKK7 or MEKK1 enhances IL-1β-induced TLR2 up-regulation in HeLa cells. Values are the mean ± SD; n = 3. The symbols indicate results significantly different (p < 0.01) from unstimulated condition (*) and IL-1β-stimulated condition (without dominant negative construct)(**). F, Overexpression of a dominant-negative mutant of MKK4 or MKK7 or MEKK1 also enhances IL-1β-induced TLR2 up-regulation in NHBE cells. Values are the mean ± SD; n = 3. The symbols indicate results significantly different (p < 0.01) from unstimulated condition (*) and IL-1β-stimulated condition (without dominant-negative construct)(**). For all the TLR2 mRNA measurement, cells were treated with IL-1β (10 ng/ml) for 3 h.
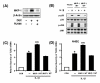
Figure 5
Glucocorticoids synergistically enhance IL-1β-induced TLR2 up-regulation via negative cross-talks with MAPK p38 and JNK1/2 pathways. A, Dexamethasone (DEX) synergistically enhances IL-1β-induced TLR2 up-regulation and RU486 counteracts the enhancing effect of DEX on IL-1β-induced TLR2 up-regulation at mRNA level in HeLa cells. HeLa cells were first pretreated with RU486 (10-6 M) for 2 h and were then treated with DEX (10-6 M) for 2 h. The cells were stimulated with IL-1β (10 ng/ml) for 3 h, and the TLR2 mRNA levels were then assessed by real-time quantitative PCR. Values are the mean ± SD; n = 3. The symbols indicate results significantly different (p < 0.01) from unstimulated condition (*) and IL-1β-DEX-stimulated condition (without RU486)(**). B, DEX (10-6 M) synergistically enhances IL-1β-induced TLR2 up-regulation and RU486 (10-6 M) counteracts the enhancing effect of DEX on IL-1β-induced TLR2 up-regulation at mRNA level in NHBE cells. Cells were first pretreated with RU486 (10-6 M) for 2 h and were then treated with DEX (10-6 M) for 2 h. The cells were stimulated with IL-1β (10 ng/ml) for 3 h, and the TLR2 mRNA levels were then assessed. Values are the mean ± SD; n = 3. The symbols indicate results significantly different (p < 0.01) from unstimulated condition (*) and IL-1β-DEX-stimulated condition (without RU486)(**). C, Glucocorticoids also synergistically enhance the induction of TLR2 at protein level in HeLa cells. HeLa cells were first treated for 2 h with DEX (10-6 M). The cells were then stimulated with IL-1β (10 ng/ml) for 5 h. Protein level of TLR2 was assessed by Western blot analysis using an anti-hTLR2 antibody. β-Actin was used as a protein loading control. D, DEX inhibits IL-1β-induced phosphorylation of p38, but not MKK3/6, and the inhibitory effect of DEX is blocked by RU486. HeLa cells were first treated with RU486 (10-6 M) for 2 h and were then treated with DEX (10-6 M) for 2 h. The cells were stimulated with IL-1β (10 ng/ml) for 20 min. IL-1β-induced phosphorylation of p38 MAPK and MKK3/6 was then assessed by Western blot analysis. E, DEX inhibits IL-1β-induced phosphorylation of JNK, but not MKK4, and the inhibitory effect of DEX is blocked by RU486. HeLa cells were first treated with RU486 (10-6 M) for 2 h and were then treated with DEX (10-6 M) for 2 h. The cells were stimulated with IL-1β (10 ng/ml) for 20 min.
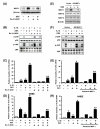
Figure 6
Glucocorticoids enhance IL-1β-induced TLR2 up-regulation likely via upregulation of MKP-1. A, MKP-1 protein expression is induced by DEX and the MKP-1 induction is counteracted by RU486 in HeLa cells. HeLa cells were first treated with RU486 (10-6 M) for 2 h and were then treated with DEX (10-6 M) for 2 h. Protein level of MKP-1 was assessed by Western blot analysis. β-Actin was used as a protein loading control. B, Overexpression of wild-type MKP-1 inhibits IL-1β-induced p38 and JNK phosphorylation, whereas overexpression of MKP-1 mutant has almost no effect on IL-1β-induced p38 and JNK phosphorylation. Transfected HeLa cells were stimulated with IL-1β (10 ng/ml) for 20 min. IL-1β-induced phosphorylation of p38 MAPK and JNK was then assessed by Western blot analysis. C, Overexpression of wild-type MKP-1 enhances the IL-1β-induced TLR2 expression, whereas overexpression of MKP-1 mutant has almost no effect on the IL-1β-induced TLR2 expression in HeLa cells. Values are the mean ± SD; n = 3. The symbols indicate results significantly different (p < 0.01) from unstimulated condition (*) and IL-1β-stimulated condition (without MKP-1 construct)(**). D, Overexpression of wild-type MKP-1 enhances the IL-1β-induced TLR2 expression, whereas overexpression of MKP-1 mutant has almost no effect on the IL-1β-induced TLR2 expression in NHBE cells. Values are the mean ± SD; n = 3. The symbols indicate results significantly different (p < 0.01) from unstimulated condition (*) and IL-1β-stimulated condition (without MKP-1 construct)(**). For all of the TLR2 mRNA measurement, cells were treated with IL-1β (10 ng/ml) for 3 h.
Figure 7
Inhibition of MKP-1 attenuates dexamethasone-mediated inhibition of IL-1β-induced p38 and JNK phosphorylation and enhancement of TLR2 expression. A, MKP-1 protein expression is induced by DEX and the MKP-1 induction is inhibited by Ro-31-8220, an inhibitor for MKP-1 expression, in HeLa cells. HeLa cells were first treated with Ro-31-8220 (10-6 M) for 2 h, and were then treated with DEX (10-6 M) for 2 h. Protein level of MKP-1 was assessed by Western blot analysis. β-Actin was used as a protein loading control. B, The inhibitory effect of DEX on IL-1β-induced p38 or JNK phosphorylation is antagonized by Ro-31-8220. HeLa cells were first treated with Ro-31-8220 (10-6 M) for 2 h and were then treated with DEX (10-6 M) for 2 h. The cells were stimulated with IL-1β (10 ng/ml) for 20 min. IL-1β-induced phosphorylation of p38 or JNK was then assessed by Western blot analysis. C, DEX-mediated enhancement of IL-1β-induced TLR2 expression is greatly inhibited by Ro-31-8220 in HeLa cells. HeLa cells were first treated with Ro-31-8220 (10-6 M) for 2 h and were then treated with DEX (10-6 M) for 2 h. The cells were stimulated with IL-1β (10 ng/ml) for 3 h, and the TLR2 mRNA levels were then assessed by real-time quantitative PCR. Values are the mean ± SD; n = 3. The symbols indicate results significantly different (p < 0.01) from unstimulated condition (*) and IL-1β-DEX-stimulated condition (without Ro-31-8220)(**). D, DEX-mediated enhancement of IL-1β-induced TLR2 expression is greatly inhibited by Ro-31-8220 in NHBE cells. NHBE cells were first treated with Ro-31-8220 (10-6 M) for 2 h and were then treated with DEX (10-6 M) for 2 h. The cells were stimulated with IL-1β (10 ng/ml) for 3 h. Values are the mean ± SD; n = 3. The symbols indicate results significantly different (p < 0.01) from unstimulated condition (*) and IL-1β-DEX-stimulated condition (without Ro-31-8220)(**). E, Overexpression of the antisense MKP-1 inhibited MKP-1 protein expression, but not MKP-2 or MKP-3 expression. Transfected HeLa cells were treated with DEX (10-6 M) for 2 h. Protein level of MKP-1, -2 and -3 were assessed by Western blot analysis. β-Actin was used as a protein loading control. F, Overexpression of antisense MKP-1 blocks the inhibitory effect of DEX on IL-1β-induced p38 or JNK phosphorylation in HeLa cells. Transfected HeLa cells were first pretreated with DEX (10-6 M) for 2 h. The cells were then stimulated with IL-1β (10 ng/ml) for 20 min. IL-1β-induced phosphorylation of p38 or JNK was next assessed by Western blot analysis. G, DEX-mediated enhancement of IL-1β-induced TLR2 expression is attenuated by overexpression of antisense MKP-1 in HeLa cells. Transfected HeLa cells were pretreated with DEX (10-6 M) for 2 h, and then stimulated with IL-1β (10 ng/ml) for 3 h. Values are the mean ± SD; n = 3. The symbols indicate results significantly different (p < 0.01) from unstimulated condition (*) and IL-1β-DEX-stimulated condition (without antisense MKP-1 construct)(**). H, DEX-mediated enhancement of IL-1β-induced TLR2 expression is also attenuated by overexpression of antisense MKP-1 in NHBE cells. Transfected cells werepretreated with DEX (10-6 M) for 2 h, and then stimulated with IL-1β (10 ng/ml) for 3 h. Values are the mean ± SD; n = 3. The symbols indicate results significantly different (p < 0.01) from unstimulated condition (*) and IL-1β-DEX-stimulated condition (without antisense MKP-1 construct)(**).
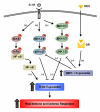
Figure 8
Schematic representation of the signaling pathways involved in glucocorticoid-mediated enhancement of IL-1β-induced TLR2 expression via MKP-1-dependent inhibition of p38 and JNK in human epithelial cells. IL-1β up-regulates TLR2 expression via a positive IKKβ-IκBα-dependent NF-κB pathway and negative MKK3/6-p38α/β and MEKK1-MKK4/7-JNK1/2 signaling pathways. Glucocorticoids synergistically enhance IL-1β-induced TLR2 expression via up-regulation of MKP-1 that, in turn, leads to dephosphorylation and inactivation of p38 and JNK pathways, the negative regulators for TLR2 expression. Abbreviations: DEX, dexamethasone; GR, glucocorticoids receptor; IκBα, Inhibitor of NF-κBα ; IKKβ, IκB kinase β ; IL-1β, interleukin 1 β ; JNK, c-Jun N-terminal kinase;MAPK, mitogen-activated protein kinase; MKK, MAPK kinase; MEKK1, MAPK/Erk kinase kinase 1; MKP-1, MAP kinase phosphatase-1; NF-κB, nuclear factor κB; TLR2, Toll-like receptor 2.
Similar articles
- Inhibition of p38 MAPK by glucocorticoids via induction of MAPK phosphatase-1 enhances nontypeable Haemophilus influenzae-induced expression of toll-like receptor 2.
Imasato A, Desbois-Mouthon C, Han J, Kai H, Cato AC, Akira S, Li JD. Imasato A, et al. J Biol Chem. 2002 Dec 6;277(49):47444-50. doi: 10.1074/jbc.M208140200. Epub 2002 Sep 27. J Biol Chem. 2002. PMID: 12356755 - Glucocorticoids synergistically enhance nontypeable Haemophilus influenzae-induced Toll-like receptor 2 expression via a negative cross-talk with p38 MAP kinase.
Shuto T, Imasato A, Jono H, Sakai A, Xu H, Watanabe T, Rixter DD, Kai H, Andalibi A, Linthicum F, Guan YL, Han J, Cato AC, Lim DJ, Akira S, Li JD. Shuto T, et al. J Biol Chem. 2002 May 10;277(19):17263-70. doi: 10.1074/jbc.M112190200. Epub 2002 Feb 26. J Biol Chem. 2002. PMID: 11867630 - Regulation of c-Jun N-terminal kinase and p38 kinase pathways in endothelial cells.
Wadgaonkar R, Pierce JW, Somnay K, Damico RL, Crow MT, Collins T, Garcia JG. Wadgaonkar R, et al. Am J Respir Cell Mol Biol. 2004 Oct;31(4):423-31. doi: 10.1165/rcmb.2003-0384OC. Epub 2004 Jul 1. Am J Respir Cell Mol Biol. 2004. PMID: 15231489 - Dual-specificity phosphatase 1: a critical regulator of innate immune responses.
Abraham SM, Clark AR. Abraham SM, et al. Biochem Soc Trans. 2006 Dec;34(Pt 6):1018-23. doi: 10.1042/BST0341018. Biochem Soc Trans. 2006. PMID: 17073741 Review.
Cited by
- The multiple roles of chronic stress and glucocorticoids in Alzheimer's disease pathogenesis.
Burke MR, Sotiropoulos I, Waites CL. Burke MR, et al. Trends Neurosci. 2024 Nov;47(11):933-948. doi: 10.1016/j.tins.2024.08.015. Epub 2024 Sep 21. Trends Neurosci. 2024. PMID: 39307629 Review. - Glucocorticoids and medroxyprogesterone acetate synergize with inflammatory stimuli to selectively upregulate CCL20 transcription.
Moliki JM, Nhundu TJ, Maritz L, Avenant C, Hapgood JP. Moliki JM, et al. Mol Cell Endocrinol. 2023 Mar 1;563:111855. doi: 10.1016/j.mce.2023.111855. Epub 2023 Jan 13. Mol Cell Endocrinol. 2023. PMID: 36646303 Free PMC article. - Capsule-dependent impact of MAPK signalling on host cell invasion and immune response during infection of the choroid plexus epithelium by Neisseria meningitidis.
Herold R, Scholtysik R, Moroniak S, Weiss C, Ishikawa H, Schroten H, Schwerk C. Herold R, et al. Fluids Barriers CNS. 2021 Dec 4;18(1):53. doi: 10.1186/s12987-021-00288-7. Fluids Barriers CNS. 2021. PMID: 34863201 Free PMC article. - Pleiotropic Effects of Glucocorticoids on the Immune System in Circadian Rhythm and Stress.
Shimba A, Ejima A, Ikuta K. Shimba A, et al. Front Immunol. 2021 Oct 8;12:706951. doi: 10.3389/fimmu.2021.706951. eCollection 2021. Front Immunol. 2021. PMID: 34691020 Free PMC article. Review. - Effects of the Cytoplasm and Mitochondrial Specific Hydroxyl Radical Scavengers TA293 and mitoTA293 in Bleomycin-Induced Pulmonary Fibrosis Model Mice.
Sakai T, Takagaki H, Yamagiwa N, Ui M, Hatta S, Imai J. Sakai T, et al. Antioxidants (Basel). 2021 Aug 31;10(9):1398. doi: 10.3390/antiox10091398. Antioxidants (Basel). 2021. PMID: 34573030 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- DC005843/DC/NIDCD NIH HHS/United States
- R01 DC004562/DC/NIDCD NIH HHS/United States
- R01 DC005843/DC/NIDCD NIH HHS/United States
- R01 HL070293/HL/NHLBI NIH HHS/United States
- HL070293/HL/NHLBI NIH HHS/United States
- DC004562/DC/NIDCD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous