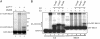The UbcH8 ubiquitin E2 enzyme is also the E2 enzyme for ISG15, an IFN-alpha/beta-induced ubiquitin-like protein - PubMed (original) (raw)
The UbcH8 ubiquitin E2 enzyme is also the E2 enzyme for ISG15, an IFN-alpha/beta-induced ubiquitin-like protein
Chen Zhao et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Ubiquitin-(Ub) like proteins (Ubls) are conjugated to their targets by an enzymatic cascade involving an E1 activating enzyme, an E2 conjugating enzyme, and in some cases an E3 ligase. ISG15 is a Ubl that is conjugated to cellular proteins after IFN-alpha/beta stimulation. Although the E1 enzyme for ISG15 (Ube1L/E1(ISG15)) has been identified, the identities of the downstream components of the ISG15 conjugation cascade have remained elusive. Here we report the purification of an E2 enzyme for ISG15 and demonstrate that it is UbcH8, an E2 that also functions in Ub conjugation. In vitro assays with purified Ub E2 enzymes and in vivo RNA interference assays indicate that UbcH8 is a major E2 enzyme for ISG15 conjugation. These results indicate that the ISG15 conjugation pathway overlaps or converges with the Ub conjugation pathway at the level of a specific E2 enzyme. Furthermore, these results raise the possibility that the ISG15 conjugation pathway might use UbcH8-competent Ub ligases in vivo. As an initial test of this hypothesis, we have shown that a UbcH8-competent Ub ligase conjugates ISG15 to a specific target in vitro. These results challenge the concept that Ub and Ubl conjugation pathways are strictly parallel and nonoverlapping and have important implications for understanding the regulation and function of ISG15 conjugation in the IFN-alpha/beta response.
Figures
Fig. 1.
Identification of the E2 enzyme for ISG15 conjugation. (A) Extracts from IFN-β-treated (lanes 3 and 4) or untreated (lanes 1 and 2) A549 cells were incubated with 32P-ISG15 protein in the absence (lanes 1 and 3) or presence (lanes 2 and 4) of E1ISG15 for 30 min at 25°C. The protein products were resolved by electrophoresis on a 10% polyacrylamide gel. Positions of molecular mass markers are shown on the right. (B)Affinity purification of the E2 enzyme for ISG15. Cell extract (15 mg of protein) from IFN-β-treated (lanes 1 and 3) or untreated (lane 2) A549 cells were incubated with GST-ISG15 (2 mg) in either the absence (lane 1) or presence (lanes 2 and 3) of E1ISG15 (500 μg) in a final volume of 1 ml for 30 min at 25°C under the thioester reaction conditions described in Methods. The reaction products were affinity selected on glutathione-Sepharose, and thioester bonds were cleaved by DTT treatment. The eluted proteins were subjected to electrophoresis on 15% polyacrylamide gels, followed by Coomassie blue staining. The 17-kDa protein in lane 3 was digested with trypsin, and the smallest tryptic peptide was sequenced by automated Edman degradation at the W. M. Keck Foundation Biotechnology Resource Laboratory at Yale University.
Fig. 2.
UbcH8 functions as a major E2 enzyme for ISG15 in vitro. (A) 32P-GST-ISG15 was incubated in the presence (lanes 1 and 3) or absence (lane 2) of UbcH8 and in the presence (lanes 2 and 3) or absence (lane 1) of E1ISG15. (B) Either 32P-GST-Ub (lanes 1-5) or 32P-GST-ISG15 (lanes 6-10) was incubated in the absence (lanes 1 and 6) or presence of E1Ub (lanes 2-5) or E1ISG15 (lanes 7-10) and in the absence or presence of the indicated E2 proteins.
Fig. 4.
A Ub E3 functions with ISG15 in vitro. (A) Purified FLAG-WBP2 protein was incubated with either Ub, E1Ub, and UbcH8 (lanes 1-3) or ISG15, E1ISG15, and UbcH8 (lanes 4-6), in the absence (lanes 1 and 4) or presence of Rsp5p (lanes 2 and 5) or in the presence the C-A mutant of Rsp5p (lanes 3 and 6). Reactions products were analyzed by immunoblotting with anti-FLAG antibody. (B) Conjugation reactions were performed as in A, in the presence of 32P-Ub (lanes 1 and 2) or 32P-ISG15 (lanes 3 and 4). Reaction products were immunoprecipitated with anti-FLAG antibody and analyzed by SDS/PAGE and autoradiography.
Fig. 3.
UbcH8 functions as a major E2 enzyme for ISG15 in vivo. Cells were either mock transfected (-siRNA) or transfected with an siRNA (20 nM final concentration) directed against bases 28-49 of the ORF of human UbcH8 mRNA (+siRNA lanes). After 24 h, the cells were left untreated (-IFN lanes) or were treated with IFN-β (1,000 units/ml; +IFN lanes). After another 24 h, the cells were collected. RNA was analyzed for UbcH8 mRNA by Northern analysis (Left), and proteins were analyzed by immunoblotting with ISG15 antiserum (Right). Each lane of the Northern blot contained 12 μg of total RNA, and the presence of equal amounts of RNA in each lane was confirmed by ethidium bromide staining of 28S ribosomal RNA (not shown). The same results were obtained by using a second siRNA that was directed against bases 239-258 of the ORF of UbcH8 mRNA, and an siRNA directed against a sequence in the mRNA for GFP did not decrease either UbcH8 mRNA or ISG15 conjugation (not shown).
Similar articles
- The basis for selective E1-E2 interactions in the ISG15 conjugation system.
Durfee LA, Kelley ML, Huibregtse JM. Durfee LA, et al. J Biol Chem. 2008 Aug 29;283(35):23895-902. doi: 10.1074/jbc.M804069200. Epub 2008 Jun 26. J Biol Chem. 2008. PMID: 18583345 Free PMC article. - Link between the ubiquitin conjugation system and the ISG15 conjugation system: ISG15 conjugation to the UbcH6 ubiquitin E2 enzyme.
Takeuchi T, Iwahara S, Saeki Y, Sasajima H, Yokosawa H. Takeuchi T, et al. J Biochem. 2005 Dec;138(6):711-9. doi: 10.1093/jb/mvi172. J Biochem. 2005. PMID: 16428300 - Herc5, an interferon-induced HECT E3 enzyme, is required for conjugation of ISG15 in human cells.
Dastur A, Beaudenon S, Kelley M, Krug RM, Huibregtse JM. Dastur A, et al. J Biol Chem. 2006 Feb 17;281(7):4334-8. doi: 10.1074/jbc.M512830200. Epub 2005 Dec 28. J Biol Chem. 2006. PMID: 16407192 - Coronaviral PLpro proteases and the immunomodulatory roles of conjugated versus free Interferon Stimulated Gene product-15 (ISG15).
Gold IM, Reis N, Glaser F, Glickman MH. Gold IM, et al. Semin Cell Dev Biol. 2022 Dec;132:16-26. doi: 10.1016/j.semcdb.2022.06.005. Epub 2022 Jun 25. Semin Cell Dev Biol. 2022. PMID: 35764457 Free PMC article. Review. - ISG15 and immune diseases.
Jeon YJ, Yoo HM, Chung CH. Jeon YJ, et al. Biochim Biophys Acta. 2010 May;1802(5):485-96. doi: 10.1016/j.bbadis.2010.02.006. Epub 2010 Feb 12. Biochim Biophys Acta. 2010. PMID: 20153823 Free PMC article. Review.
Cited by
- ISG15 modification of the eIF4E cognate 4EHP enhances cap structure-binding activity of 4EHP.
Okumura F, Zou W, Zhang DE. Okumura F, et al. Genes Dev. 2007 Feb 1;21(3):255-60. doi: 10.1101/gad.1521607. Genes Dev. 2007. PMID: 17289916 Free PMC article. - ISG15: its roles in SARS-CoV-2 and other viral infections.
Sarkar L, Liu G, Gack MU. Sarkar L, et al. Trends Microbiol. 2023 Dec;31(12):1262-1275. doi: 10.1016/j.tim.2023.07.006. Epub 2023 Aug 10. Trends Microbiol. 2023. PMID: 37573184 Free PMC article. Review. - Mice lacking the ISG15 E1 enzyme UbE1L demonstrate increased susceptibility to both mouse-adapted and non-mouse-adapted influenza B virus infection.
Lai C, Struckhoff JJ, Schneider J, Martinez-Sobrido L, Wolff T, García-Sastre A, Zhang DE, Lenschow DJ. Lai C, et al. J Virol. 2009 Jan;83(2):1147-51. doi: 10.1128/JVI.00105-08. Epub 2008 Nov 12. J Virol. 2009. PMID: 19004958 Free PMC article. - Type I IFN induces protein ISGylation to enhance cytokine expression and augments colonic inflammation.
Fan JB, Miyauchi-Ishida S, Arimoto K, Liu D, Yan M, Liu CW, Győrffy B, Zhang DE. Fan JB, et al. Proc Natl Acad Sci U S A. 2015 Nov 17;112(46):14313-8. doi: 10.1073/pnas.1505690112. Epub 2015 Oct 29. Proc Natl Acad Sci U S A. 2015. PMID: 26515094 Free PMC article. - Snai2 and Snai3 transcriptionally regulate cellular fitness and functionality of T cell lineages through distinct gene programs.
Pioli PD, Whiteside SK, Weis JJ, Weis JH. Pioli PD, et al. Immunobiology. 2016 May;221(5):618-33. doi: 10.1016/j.imbio.2016.01.007. Epub 2016 Jan 22. Immunobiology. 2016. PMID: 26831822 Free PMC article.
References
- Levy, D. E. & Garcia-Sastre, A. (2001) Cytokine Growth Factor Rev. 12, 143-156. - PubMed
- Haas, A. L., Ahrens, P., Bright, P. M. & Ankel, H. (1987) J. Biol. Chem. 262, 11315-11323. - PubMed
- Farrell, P. J., Broeze, R. J. & Lengyel, P. (1979) Nature 279, 523-525. - PubMed
- Loeb, K. R. & Haas, A. L. (1992) J. Biol. Chem. 267, 7806-7813. - PubMed
- Schwartz, D. C. & Hochstrasser, M. (2003) Trends Biochem. Sci. 28, 321-328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA72943/CA/NCI NIH HHS/United States
- AI17772/AI/NIAID NIH HHS/United States
- R01 CA072943/CA/NCI NIH HHS/United States
- R37 GM069530/GM/NIGMS NIH HHS/United States
- R01 GM069530/GM/NIGMS NIH HHS/United States
- GM69530/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous