Molecular evidence links cryptic diversification in polar planktonic protists to Quaternary climate dynamics - PubMed (original) (raw)
Molecular evidence links cryptic diversification in polar planktonic protists to Quaternary climate dynamics
Kate F Darling et al. Proc Natl Acad Sci U S A. 2004.
Abstract
It is unknown how pelagic marine protists undergo diversification and speciation. Superficially, the open ocean appears homogeneous, with few clear barriers to gene flow, allowing extensive, even global, dispersal. Yet, despite the apparent lack of opportunity for genetic isolation, diversity is prevalent within marine taxa. A lack of candidate isolating mechanisms would seem to favor sympatric over allopatric speciation models to explain the diversity and biogeographic patterns observed in the oceans today. However, the ocean is a dynamic system, and both current and past circulation patterns must be considered in concert to gain a true perspective of gene flow through time. We have derived a comprehensive picture of the mechanisms potentially at play in the high latitudes by combining molecular, biogeographic, fossil, and paleoceanographic data to reconstruct the evolutionary history of the polar planktonic foraminifer Neogloboquadrina pachyderma sinistral. We have discovered extensive genetic diversity within this morphospecies and that its current "extreme" polar affinity did not appear until late in its evolutionary history. The molecular data demonstrate a stepwise progression of diversification starting with the allopatric isolation of Atlantic Arctic and Antarctic populations after the onset of the Northern Hemisphere glaciation. Further diversification occurred only in the Southern Hemisphere and seems to have been linked to glacial-interglacial climate dynamics. Our findings demonstrate the role of Quaternary climate instability in shaping the modern high-latitude plankton. The divergent evolutionary history of N. pachyderma sinistral genotypes implies that paleoceanographic proxies based on this taxon should be calibrated independently.
Figures
Fig. 1.
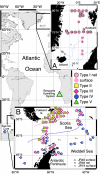
Sampling localities and distribution of the high-latitude and Benguela left-coiling (sin) N. pachyderma SSU genotypes (n = 566). (Inset A) Distribution pattern of N. pachyderma (sin) type I (n = 216) in the Fram Strait and Norwegian Sea. The contours denote the sediment “core-top” coiling ratio (% sin coiling), delineating the modern N. pachyderma (sin) province (11). (Inset B) Distribution pattern of N. pachyderma (sin) types II (n = 41), III (n = 117), and IV (n = 79) in the subpolar/polar Antarctic. The pink contour delineates the approximate position in austral summer of 2000 of the Sub-antarctic Front and the blue contour of the Polar Front as deduced from its average position (12) and from sea-surface temperature profiles generated during cruise JR48. The main map shows the location of the N. pachyderma (sin) type IV samples (n = 3) collected from the Bellingshausen Sea ice core and the location of the type V samples (n = 22) collected from the Benguela upwelling system.
Fig. 2.
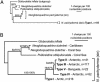
SSU rDNA phylogenetic trees highlighting the evolutionary relationships among the Neogloboquadrinidae. Trees were constructed both including (A) (685 sites, NJ) and excluding (B) (835 sites, ML) the highly divergent right-coiling (dextral) N. pachyderma SSU genotype. G. inflata was used as an outgroup. Bootstrap values (NJ/ML; expressed as a percentage) indicate support for branches in the trees.
Fig. 3.
Sequence alignment showing the extensive variation in the first variable region of the SSU rRNA gene fragment. The variable region is shown in normal text. Conserved regions, alignable across all neogloboquadrinid and outgroup taxa and used in phylogenetic tree construction (Fig. 2), are shown in bold type (∼ indicates sequences not displayed; -indicates gaps introduced in aligning sequences). Additional differences are observed between SSU genotypes in other variable regions of the SSU rDNA fragment.
Fig. 4.
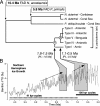
(A) PL chronogram (29) with branches proportional to time. The boxed divergence times are based on the foraminiferal fossil record (27) (FAD indicates first appearance datum). Estimates of divergence times of the cryptic divergences within N. pachyderma (sin) are indicated in italic type. (B) These estimates can be compared with a proxy record for global ice volume (30). Major intervals of ice-sheet growth in the Northern Hemisphere are indicated with dashed arrows (31).
Similar articles
- Global molecular phylogeography reveals persistent Arctic circumpolar isolation in a marine planktonic protist.
Darling KF, Kucera M, Wade CM. Darling KF, et al. Proc Natl Acad Sci U S A. 2007 Mar 20;104(12):5002-7. doi: 10.1073/pnas.0700520104. Epub 2007 Mar 13. Proc Natl Acad Sci U S A. 2007. PMID: 17360336 Free PMC article. - Cryptic species of planktonic foraminifera: their effect on palaeoceanographic reconstructions.
Kucera M, Darling KF. Kucera M, et al. Philos Trans A Math Phys Eng Sci. 2002 Apr 15;360(1793):695-718. doi: 10.1098/rsta.2001.0962. Philos Trans A Math Phys Eng Sci. 2002. PMID: 12804300 - Palaeoceanographic implications of genetic variation in living North Atlantic Neogloboquadrina pachyderma.
Bauch D, Darling K, Simstich J, Bauch HA, Erlenkeuser H, Kroon D. Bauch D, et al. Nature. 2003 Jul 17;424(6946):299-302. doi: 10.1038/nature01778. Nature. 2003. PMID: 12867978 - Diversity and ecology of Radiolaria in modern oceans.
Biard T. Biard T. Environ Microbiol. 2022 May;24(5):2179-2200. doi: 10.1111/1462-2920.16004. Epub 2022 Apr 24. Environ Microbiol. 2022. PMID: 35412019 Free PMC article. Review. - The adaptation of polar fishes to climatic changes: Structure, function and phylogeny of haemoglobin.
Verde C, Giordano D, di Prisco G. Verde C, et al. IUBMB Life. 2008 Jan;60(1):29-40. doi: 10.1002/iub.1. IUBMB Life. 2008. PMID: 18379990 Review.
Cited by
- Long-range dispersal and high-latitude environments influence the population structure of a "stress-tolerant" dinoflagellate endosymbiont.
Pettay DT, Lajeunesse TC. Pettay DT, et al. PLoS One. 2013 Nov 5;8(11):e79208. doi: 10.1371/journal.pone.0079208. eCollection 2013. PLoS One. 2013. PMID: 24223906 Free PMC article. - Intraspecific Variation in Protists: Clues for Microevolution from Poteriospumella lacustris (Chrysophyceae).
Majda S, Boenigk J, Beisser D. Majda S, et al. Genome Biol Evol. 2019 Sep 1;11(9):2492-2504. doi: 10.1093/gbe/evz171. Genome Biol Evol. 2019. PMID: 31384914 Free PMC article. - Ecological partitioning and diversity in tropical planktonic foraminifera.
Seears HA, Darling KF, Wade CM. Seears HA, et al. BMC Evol Biol. 2012 Apr 16;12:54. doi: 10.1186/1471-2148-12-54. BMC Evol Biol. 2012. PMID: 22507289 Free PMC article. - Planktonic foraminifera genomic variations reflect paleoceanographic changes in the Arctic: evidence from sedimentary ancient DNA.
Pawłowska J, Wollenburg JE, Zajączkowski M, Pawlowski J. Pawłowska J, et al. Sci Rep. 2020 Sep 15;10(1):15102. doi: 10.1038/s41598-020-72146-9. Sci Rep. 2020. PMID: 32934321 Free PMC article. - A solution for constraining past marine Polar Amplification.
Morley A, de la Vega E, Raitzsch M, Bijma J, Ninnemann U, Foster GL, Chalk TB, Meilland J, Cave RR, Büscher JV, Kucera M. Morley A, et al. Nat Commun. 2024 Oct 18;15(1):9002. doi: 10.1038/s41467-024-53424-w. Nat Commun. 2024. PMID: 39424833 Free PMC article.
References
- Finlay, B. J. (2002) Science 296, 1061-1063. - PubMed
- Darling, K. F., Wade, C. M., Steward, I. A., Kroon, D., Dingle, R. & Leigh Brown, A. J. (2000) Nature 405, 43-47. - PubMed
- Palumbi, S. R. (1994) Annu. Rev. Ecol. Syst. 25, 547-572.
- Norris, R. D. (2000) Paleobiology 26, Suppl., 236-258.
- Knowlton, N. (1993) Annu. Rev. Ecol. Syst. 24, 189-216.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous