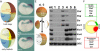Neural induction in Xenopus: requirement for ectodermal and endomesodermal signals via Chordin, Noggin, beta-Catenin, and Cerberus - PubMed (original) (raw)
Neural induction in Xenopus: requirement for ectodermal and endomesodermal signals via Chordin, Noggin, beta-Catenin, and Cerberus
Hiroki Kuroda et al. PLoS Biol. 2004 May.
Abstract
The origin of the signals that induce the differentiation of the central nervous system (CNS) is a long-standing question in vertebrate embryology. Here we show that Xenopus neural induction starts earlier than previously thought, at the blastula stage, and requires the combined activity of two distinct signaling centers. One is the well-known Nieuwkoop center, located in dorsal-vegetal cells, which expresses Nodal-related endomesodermal inducers. The other is a blastula Chordin- and Noggin-expressing (BCNE) center located in dorsal animal cells that contains both prospective neuroectoderm and Spemann organizer precursor cells. Both centers are downstream of the early beta-Catenin signal. Molecular analyses demonstrated that the BCNE center was distinct from the Nieuwkoop center, and that the Nieuwkoop center expressed the secreted protein Cerberus (Cer). We found that explanted blastula dorsal animal cap cells that have not yet contacted a mesodermal substratum can, when cultured in saline solution, express definitive neural markers and differentiate histologically into CNS tissue. Transplantation experiments showed that the BCNE region was required for brain formation, even though it lacked CNS-inducing activity when transplanted ventrally. Cell-lineage studies demonstrated that BCNE cells give rise to a large part of the brain and retina and, in more posterior regions of the embryo, to floor plate and notochord. Loss-of-function experiments with antisense morpholino oligos (MO) showed that the CNS that forms in mesoderm-less Xenopus embryos (generated by injection with Cerberus-Short [CerS] mRNA) required Chordin (Chd), Noggin (Nog), and their upstream regulator beta-Catenin. When mesoderm involution was prevented in dorsal marginal-zone explants, the anterior neural tissue formed in ectoderm was derived from BCNE cells and had a complete requirement for Chd. By injecting Chd morpholino oligos (Chd-MO) into prospective neuroectoderm and Cerberus morpholino oligos (Cer-MO) into prospective endomesoderm at the 8-cell stage, we showed that both layers cooperate in CNS formation. The results suggest a model for neural induction in Xenopus in which an early blastula beta-Catenin signal predisposes the prospective neuroectoderm to neural induction by endomesodermal signals emanating from Spemann's organizer.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures
Figure 6. Anterior Neural Induction in Keller Explants Requires Chd
(A) Proposed vertical and planar signals in neural induction (following Ruiz i Altaba 1993). (B) Diagram of Keller explant preparation and subsequent elongation of the endomesoderm by convergent extension (Keller 1991). (C) The neural and mesodermal regions of Keller explants contain descendants of BCNE cells (in blue) marked by blastomere injection at the 64-cell stage. (D) Expression of Otx2 and Krox20 in Keller explants (n = 7). (E) Injection of 17 ng _Chd-_MO completely blocked Otx2 and Krox20 expression in neural regions, while expression of Otx2 in anterior endoderm was not affected (n = 10). (F) The differentiated neuron marker N-tubulin is expressed in Keller explants (n = 8). (G) Partial inhibition of N-tubulin by injection of _Chd-_MO (n = 7). (H and I) Summary of the effects of _Chd-_MO in Keller explants. Abbreviations: SC, spinal cord; CG, cement gland; Epi, epidermis. (J) RT-PCR analyses of the effect of _Chd-_MO in Keller explants; samples injected with (plus) or without (minus) _Chd-_MO are indicated. Lane 1, whole embryos; lanes 2–7, Keller sandwiches. Note that expression of the neural markers NCAM and N-tubulin in Keller sandwiches was abolished by co-injection with 200 pg of dnFGF receptor 4a (dnFGF4a) mRNA and 17 ng of _Chd-_MO (lane 5). Injection with 600 pg of CerS mRNA, which eliminates mesoderm but not BCNE formation, does not affect neural induction in this assay (lane 6).
Figure 1. Two Signaling Centers Coexist in the Xenopus Blastula
(A) Diagram of early events between 1-cell stage and early blastula. (B–D) Expression of Chd, Nog, and Xnr3 transcripts just after midblastula transition (7 h postfertilization). Embryos were hybridized as whole mounts, stored in methanol for 1 mo at room temperature to improve contrast, and sectioned with a razor blade. (E) RT-PCR analysis of gene markers at midblastula, early stage 9. Six samples were prepared by dissections of blastula regions as shown in the diagram. (F) Summary of gene expression at blastula. The BCNE center expresses Chd, Nog, Siamois, and Xnr3, while the Nieuwkoop center expresses Xnr2, Xnr6, and Cer.
Figure 2. The BCNE Center Contributes to Forebrain and Midline Structures
(A) Method used for lineage tracing of the BCNE center with biotin-dextran amine (BDA) labeled grafts. (B) Sagittal section of a recently grafted BCNE at stage 9. (C) Chd mRNA expression at stage 9. (D) BCNE descendants at stage 10.5. (E) Chd mRNA expression at stage 10.5. (F) BCNE center descendants at stage 11. (G) Dorsal view of BCNE descendants at neural plate stage 14. (H) Double staining of transplanted BCNE region with nuclear lacZ mRNA and epidermal ectoderm of the host with epidermal cytokeratin (epi) probe in light red at stage 14. (I) Transverse section at the level of the trunk at stage 16. Abbreviations: fp, floor plate; no, notochord. (J–L) Transverse sections at stage 40. Abbreviations: fp, floor plate; hb, hindbrain; he, heart; le, lens; mb, midbrain; no, notochord; ov, otic vesicle; re, retina. (M) Dorsal view of 6-d embryo transplanted with a BCNE graft from CMV-GFP transgenic embryos. Abbreviations: br, brain; fp, floor plate; on, optic nerve; op, olfactory placode. (N) Side view at 4 d showing labeled retina and brain. Abbreviation: br, brain.
Figure 3. The Blastula Dorsal Animal Cap Is Specified to Form CNS
(A) Experimental diagram showing embryos injected with CerS mRNA from which three regions of the animal cap were dissected at blastula, cultured until stage 26, and processed for RT-PCR. The size of the explants was 0.3 mm by 0.3 mm in these samples. Abbreviations: A, animal pole; D, dorsal region; V, ventral animal cap. (B) RT-PCR analysis of animal cap fragments; note that anterior brain markers were expressed in the dorsal fragments in the absence of mesoderm (α-actin) and endoderm (endodermin, Edd) differentiation. Abbreviations: A, animal pole; D, dorsal region; V, ventral animal cap. (C) Experimental diagram of the small animal cap sandwich experiments; these embryos were not injected with CerS. In this case, the size of the explants was 0.15 mm by 0.15 mm leaving a 0.15-mm gap from the floor of the blastocoel to avoid contamination from mesoderm-forming cells. Fragments from two explants were sandwiched together (explants are too small to heal by curling up) and cultured in 1× Steinberg's solution until stage 40. Abbreviations: VSW, ventral sandwich; DSW, dorsal sandwich. (D) Histological section of dorsal animal cap explant (dorsal sandwich). These sandwiches differentiated into histotypic forebrain tissue including white and gray matter (4/17). Abbrevations: DSW, dorsal sandwich; gm, gray matter; wm, white matter. (E) Histological section of a ventral animal cap sandwich. All sandwiches differentiated into atypical epidermis (n = 20). Abbreviations: ae, atypical epidermis; VSW, ventral sandwich.
Figure 4. The Dorsal Animal Cap Is Required for Brain Formation
(A) Ventral animal cap deletion (ΔV) produces a normal embryo. (B–F) Dorsal animal cap deletion (ΔD) results in loss of anterior brain structure. The headless phenotype of dorsal animal cap deletions was rescued by dorsal animal cap grafts (C) and animal pole grafts obtained from LiCl-treated embryos (E), but not by ventral animal cap transplants (D) or animal pole transplants (F). The average dorso-anterior indices (DAI) were 4.89 ([A] n = 28), 3.52 ([B] n = 25), 4.90 ([C] n = 10), 3.63 ([D] n = 19), 4.90 ([E] n = 12), and 3.50 ([F] n = 10). (G) Transplantation of the dorsal animal cap into the ventral animal cap region of a host embryo induced weak secondary axes (65.4%, n = 26). The embryo shown here was one of the strongest axes obtained. (H) Activity of BCNE transplanted ventrally was blocked by _Chd-_MO (n = 15).
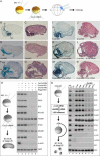
Figure 5. The CNS of Mesodermless Embryos Derives from BCNE Cells and Requires Chd, Nog, and β-Catenin
(A) Experimental design. Embryos in which mesoderm induction was inhibited (by injection of 600 pg of CerS mRNA into the vegetal pole) were sectioned at stage 38 and stained with hematoxylin-eosin or for microinjected BDA lineage tracer marking the BCNE region. (B and C) Embryos injected with CerS mRNA alone (n = 40). Abbreviation: br, brain. (D and E) Embryos injected with 17 ng of _Chd-_MO in addition to CerS (n = 21). Abbreviation: epi, epidermis. (F and G) Coinjection of 17 ng of _Chd-_MO and CerS, followed by 100 pg of Chd mRNA together with the lineage tracer (n = 19). Abbreviation: br, brain. (H) Expression of anterior CNS markers in mesodermless embryos requires Chd and Nog. RT-PCR analysis of CerS mRNA–injected embryos at tailbud stage 26. Markers of anterior brain (Otx2), eye (Rx2a), midhindbrain boundary (En2), hindbrain (Krox20), and cement gland (XAG) were inhibited by injection of _Chd-_MO, _Nog-_MO, or both. A pan-neural marker (NCAM) and a neuronal marker (N-tubulin) were partially inhibited, and the posterior neural marker HoxB9 was not affected. α-actin serves as a mesoderm marker to show that CerS blocked mesoderm in these embryos and ODC as mRNA loading control. The effects of the _Nog-_MO described here can be rescued by full-length Nog mRNA lacking the 5′ leader sequence targeted by the antisense morpholino (data not shown). (I and J) _β-cat-_MO (13.6 ng) together with CerS mRNA (n = 15). Abbreviation: epi, epidermis. (K and L) Rescue of _β-cat-_MO by 800 pg of β-catenin mRNA. Abbreviation: br, brain. (M and N) Rescue of the _β-cat-_MO phenotype by 100 pg of Chd mRNA (n = 8). (O) Chd is required for the anterior neural induction caused by β-Catenin. Neural and cement gland markers were induced in animal cap explants by activation of β-Catenin signal by the injection of 600 pg β-catenin mRNA, dnGSK3 mRNA, or LiCl treatment (lanes 3–5). Markers of anterior brain (Six3, Otx2), eye (Rx2a), midhindbrain boundary (En2), hindbrain (Krox20), and cement gland (XAG) were inhibited by _Chd-_MO (lanes 6–8). Although inhibition was not detected for the posterior neural marker HoxB9 and the pan-neural marker NCAM, the neuronal marker N-tubulin was inhibited. α-actin and α-globin are dorsal and ventral mesoderm markers, respectively, used to show the absence of mesoderm formation, and ODC serves as loading control.
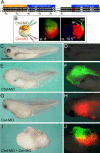
Figure 7. A Double-Assurance Mechanism in Xenopus Neural Induction That Requires Chordin and Cerberus
(A) A new _Cer-_MO is complementary to both Cer pseudoalleles, while two MOs reported by other authors (Hino et al. 2003; Silva et al. 2003) match only one allele, having three or four mismatches, respectively, with the other allele. The _Cer-_MO used in the present study inhibits head formation in intact embryos (data not shown), while the other two do not (Hino et al. 2003; Silva et al. 2003). (B) Experimental procedure and cell lineages at 32-cell and early gastrula (stage 10.5) for dorsal-animal (FDA, green) and dorsal-vegetal (TRDA, red) blastomeres microinjected at the 8-cell stage. (C and D) Uninjected embryos. (E and F) Dorsal-animal injection with 8.5 ng of _Chd-_MO alone partially inhibited head formation; green fluorescence was seen in anterior CNS. (G and H) Dorsal-vegetal injection with 17 ng of _Cer-_MO also inhibited brain formation partially; red fluorescence may be seen in anterior endomesoderm. (I and J) Injection with 8.5 ng _Chd-_MO dorsal-animally and 17 ng _Cer-_MO dorsal-vegetally blocked brain formation, but not spinal cord and somites (histological sections not shown).
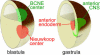
Figure 8. Double-Assurance Model for Brain Formation by the BCNE and Nieuwkoop Centers
Blastula Chd- and _Nog-_expressing cells are located in the dorsal animal region, while the Nieuwkoop center is found in the dorsal-vegetal region. At gastrula, the anterior endoderm derived from the Nieuwkoop center is found in close apposition to the prospective anterior CNS. See text for discussion.
Similar articles
- Dorsal-ventral patterning and neural induction in Xenopus embryos.
De Robertis EM, Kuroda H. De Robertis EM, et al. Annu Rev Cell Dev Biol. 2004;20:285-308. doi: 10.1146/annurev.cellbio.20.011403.154124. Annu Rev Cell Dev Biol. 2004. PMID: 15473842 Free PMC article. Review. - Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.
Elwenspoek MM, Thom H, Sheppard AL, Keeney E, O'Donnell R, Jackson J, Roadevin C, Dawson S, Lane D, Stubbs J, Everitt H, Watson JC, Hay AD, Gillett P, Robins G, Jones HE, Mallett S, Whiting PF. Elwenspoek MM, et al. Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article. - Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.
Lee CC, Chen CW, Yen HK, Lin YP, Lai CY, Wang JL, Groot OQ, Janssen SJ, Schwab JH, Hsu FM, Lin WH. Lee CC, et al. Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924 - Spatiotemporal requirements of nuclear β-catenin define early sea urchin embryogenesis.
Lhomond G, Schubert M, Croce J. Lhomond G, et al. PLoS Biol. 2024 Nov 12;22(11):e3002880. doi: 10.1371/journal.pbio.3002880. eCollection 2024 Nov. PLoS Biol. 2024. PMID: 39531468 Free PMC article.
Cited by
- Nodal and <i>churchill1</i> position the expression of a notch ligand during <i>Xenopus</i> germ layer segregation.
Favarolo MB, Revinski DR, Garavaglia MJ, López SL. Favarolo MB, et al. Life Sci Alliance. 2022 Sep 30;5(12):e202201693. doi: 10.26508/lsa.202201693. Life Sci Alliance. 2022. PMID: 36180230 Free PMC article. - Follistatin-like-1, a diffusible mesenchymal factor determines the fate of epithelium.
Umezu T, Yamanouchi H, Iida Y, Miura M, Tomooka Y. Umezu T, et al. Proc Natl Acad Sci U S A. 2010 Mar 9;107(10):4601-6. doi: 10.1073/pnas.0909501107. Epub 2010 Feb 22. Proc Natl Acad Sci U S A. 2010. PMID: 20176958 Free PMC article. - Molecular analysis of a self-organizing signaling pathway for Xenopus axial patterning from egg to tailbud.
Azbazdar Y, De Robertis EM. Azbazdar Y, et al. Proc Natl Acad Sci U S A. 2024 Jul 9;121(28):e2408346121. doi: 10.1073/pnas.2408346121. Epub 2024 Jul 5. Proc Natl Acad Sci U S A. 2024. PMID: 38968117 Free PMC article. - Dorsal-ventral patterning and neural induction in Xenopus embryos.
De Robertis EM, Kuroda H. De Robertis EM, et al. Annu Rev Cell Dev Biol. 2004;20:285-308. doi: 10.1146/annurev.cellbio.20.011403.154124. Annu Rev Cell Dev Biol. 2004. PMID: 15473842 Free PMC article. Review. - Calcium signalling during neural induction in Xenopus laevis embryos.
Moreau M, Néant I, Webb SE, Miller AL, Leclerc C. Moreau M, et al. Philos Trans R Soc Lond B Biol Sci. 2008 Apr 12;363(1495):1371-5. doi: 10.1098/rstb.2007.2254. Philos Trans R Soc Lond B Biol Sci. 2008. PMID: 18198153 Free PMC article.
References
- Bachiller D, Klingensmith J, Kemp C, Belo JA, Anderson RM, et al. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403:658–661. - PubMed
- Bauer DV, Huang S, Moody SA. The cleavage stage origin of Spemann's organizer: Analysis of the movements of blastomere clones before and during gastrulation in Xenopus . Development. 1994;120:1179–1189. - PubMed
- Beddington RS, Robertson EJ. Axis development and early asymmetry in mammals. Cell. 2000;96:195–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HD021502/HD/NICHD NIH HHS/United States
- R37 HD021502/HD/NICHD NIH HHS/United States
- R37 HD021502-18/HD/NICHD NIH HHS/United States
- HD21502-18/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous