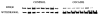Alterations in ionotropic glutamate receptor subunits during binge cocaine self-administration and withdrawal in rats - PubMed (original) (raw)
Alterations in ionotropic glutamate receptor subunits during binge cocaine self-administration and withdrawal in rats
Wenxue Tang et al. J Neurochem. 2004 May.
Abstract
Chronic cocaine use in humans and animal models is known to lead to pronounced alterations in glutamatergic function in brain regions associated with reinforcement. Previous studies have examined ionotropic glutamate receptor (iGluR) subunit protein level changes following acute and chronic experimenter-administered cocaine or after withdrawal periods from experimenter-administered cocaine. To evaluate whether alterations in expression of iGluRs are associated with cocaine reinforcement, protein levels were assessed after binge (8 h/day, 15 days; 24-h access, days 16-21) cocaine self-administration and following 2 weeks of abstinence from this binge. Western blotting was used to compare levels of iGluR protein expression (NR1-3B, GluR1-7, KA2) in the ventral tegmental area (VTA), substantia nigra (SN), nucleus accumbens (NAc), striatum and prefrontal cortex (PFC) of rats. iGluR subunits were altered in a time-dependent manner in all brain regions studied; however, selective alterations in certain iGluR subtypes appeared to be associated with binge cocaine self-administration and withdrawal in a region-specific manner. In the SN and VTA, alterations in iGluR protein levels compared with controls occurred only following binge access, whereas in the striatum and PFC, iGluR alterations occurred with binge access and following withdrawal. In the NAc, GluR2/3 levels were increased following withdrawal compared with binge access, and were the only changes observed in this region. Because subunit composition determines the functional properties of iGluRs, the observed changes may indicate alterations in the excitability of dopamine transmission underlying long-term biochemical and behavioral effects of cocaine.
Figures
Fig. 1
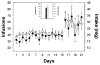
Mean ± SEM number of cocaine infusions and intake self-administered during limited and binge access periods for the two groups. Responding was engendered and maintained by intravenous cocaine infusions by both the binge (●) and withdrawal (○) groups. There was no significant difference in the number of infusions or intake between the two groups. The inset depicts mean ± SEM total number of infusions and intake for the experiments.
Fig. 2
Regional distribution of iGluR subunits in rat brain. Aliquots of 10 μg membrane fraction combined from four control rats were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotting for NMDR1 (176 kDa), NMDR2A (170 kDa), NMDAR2B (180 kDa), NMDAR3A (130 kDa), NMDAR3B (98 kDa), GluR1 (110 kDa), GluR2/3 (110 kDaA), GluR4 (110 kDa), GluR5 (105 kDa), GluR6/7 (115 kDa), KA2 (123 kDa). STR, striatum; HIPP, hippocampus.
Fig. 3
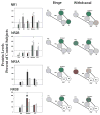
Comparisons of NMDA receptor subunit protein levels in VTA, SN, NAc, striatum and PFC following binge cocaine access (black bars) and withdrawal (grey bars). Data are mean ± SEM percentage of values for control rats in each region. Data for each subunit were statistically evaluated using one-way ANOVA. See Table 2 for significant comparisons between groups. *p < 0.05 by post-hoc analysis. Schematics of mesolimbic and nigrostriatal pathways represent graphical depiction of changes for each subunit. Green filled, increased compared with control; red filled, decreased compared with control; green diagonal bands, increased relative to binge; red diagonal bands, decreased relative to binge. N/A, not available; grey filled, no change.
Fig. 4
Comparisons of AMPA receptor subunit protein levels in VTA, SN, NAc, striatum and PFC following binge cocaine access (black bars) and withdrawal (grey bars). Data are mean ± SEM percentage of values for control rats for each antibody. Data for each subunit were statistically evaluated using one-way ANOVA. See Table 2 for significant comparisons between groups. *p < 0.05 by post-hoc analysis. Schematics of mesolimbic and nigrostriatal pathways represent graphical depiction of changes for each subunit. Green filled, increased compared with control; red filled, decreased compared with control; green diagonal bands, increased relative to binge; red diagonal bands, decreased relative to binge.
Fig. 5
Representative immunoblot of GluR2/3 protein levels in the striatum following binge cocaine access and withdrawal. Note the decrease in protein levels following binge access and the increase in levels following withdrawal. Data were analyzed by quantitative densiotometry and were found to be statistically different from control levels. Refer to Fig. 4(b) for graphic representation.
Fig. 6
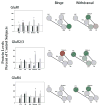
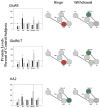
Comparisons of kainate receptor subunit protein levels in VTA, SN, NAc, striatum and PFC following binge cocaine access (black bars) and withdrawal (grey bars). Data are mean ± SEM percentage of values for control rats for each antibody. Data for each subunit were statistically evaluated using one-way ANOVA. See Table 2 for significant comparisons between groups. *p < 0.05 by post-hoc analysis. Schematics of mesolimbic and nigrostriatal pathways represent graphical depiction of changes for each subunit. Green filled, increased compared with control; red filled, decreased compared with control; green diagonal bands, increased relative to binge; red diagonal bands, decreased relative to binge.
Similar articles
- Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration.
Self DW, Choi KH, Simmons D, Walker JR, Smagula CS. Self DW, et al. Learn Mem. 2004 Sep-Oct;11(5):648-57. doi: 10.1101/lm.81404. Learn Mem. 2004. PMID: 15466321 Free PMC article. Review. - Differential regulation of ionotropic glutamate receptor subunits following cocaine self-administration.
Hemby SE, Horman B, Tang W. Hemby SE, et al. Brain Res. 2005 Dec 7;1064(1-2):75-82. doi: 10.1016/j.brainres.2005.09.051. Epub 2005 Nov 8. Brain Res. 2005. PMID: 16277980 Free PMC article. - Cocaine-induced alterations in nucleus accumbens ionotropic glutamate receptor subunits in human and non-human primates.
Hemby SE, Tang W, Muly EC, Kuhar MJ, Howell L, Mash DC. Hemby SE, et al. J Neurochem. 2005 Dec;95(6):1785-93. doi: 10.1111/j.1471-4159.2005.03517.x. J Neurochem. 2005. PMID: 16363995 Free PMC article. - Behavioral sensitization to cocaine is associated with increased glutamate receptor trafficking to the postsynaptic density after extended withdrawal period.
Ghasemzadeh MB, Mueller C, Vasudevan P. Ghasemzadeh MB, et al. Neuroscience. 2009 Mar 3;159(1):414-26. doi: 10.1016/j.neuroscience.2008.10.027. Epub 2008 Nov 1. Neuroscience. 2009. PMID: 19105975
Cited by
- Continuous exposure to the competitive N-methyl-D: -aspartate receptor antagonist, LY235959, facilitates escalation of cocaine consumption in Sprague-Dawley rats.
Allen RM, Dykstra LA, Carelli RM. Allen RM, et al. Psychopharmacology (Berl). 2007 Apr;191(2):341-51. doi: 10.1007/s00213-006-0661-3. Epub 2007 Jan 16. Psychopharmacology (Berl). 2007. PMID: 17225167 - Continuous exposure to dizocilpine facilitates escalation of cocaine consumption in male Sprague-Dawley rats.
Allen RM. Allen RM. Drug Alcohol Depend. 2014 Jan 1;134:38-43. doi: 10.1016/j.drugalcdep.2013.09.005. Epub 2013 Sep 14. Drug Alcohol Depend. 2014. PMID: 24103127 Free PMC article. - Ethanol self-administration modulation of NMDA receptor subunit and related synaptic protein mRNA expression in prefrontal cortical fields in cynomolgus monkeys.
Acosta G, Hasenkamp W, Daunais JB, Friedman DP, Grant KA, Hemby SE. Acosta G, et al. Brain Res. 2010 Mar 8;1318:144-54. doi: 10.1016/j.brainres.2009.12.050. Epub 2010 Jan 4. Brain Res. 2010. PMID: 20043891 Free PMC article. - Cytosolic proteomic alterations in the nucleus accumbens of cocaine overdose victims.
Tannu N, Mash DC, Hemby SE. Tannu N, et al. Mol Psychiatry. 2007 Jan;12(1):55-73. doi: 10.1038/sj.mp.4001914. Epub 2006 Oct 31. Mol Psychiatry. 2007. PMID: 17075605 Free PMC article. - Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration.
Self DW, Choi KH, Simmons D, Walker JR, Smagula CS. Self DW, et al. Learn Mem. 2004 Sep-Oct;11(5):648-57. doi: 10.1101/lm.81404. Learn Mem. 2004. PMID: 15466321 Free PMC article. Review.
References
- Bell K, Kalivas PW. Context-specific cross-sensitization between systemic cocaine and intra-accumbens AMPA infusion in the rat. Psychopharmacologia. 1996;127:377–383. - PubMed
- Berendse HW, Groenewegen HJ. Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. J Comp Neurol. 1990;299:187–228. - PubMed
- Berendse HW, Voorn P, te Kortschot A, Groenewegen HJ. Nuclear origin of thalamic afferents of the ventral striatum determines their relation to patch/matrix configurations in enkephalin-immunoreactivity in the rat. J Chem Neuroanat. 1988;1:3–10. - PubMed
- Bernard V, Gardiol A, Faucheux B, Bloch B, Agid Y, Hirsch EC. Expression of glutamate receptors in the human and rat basal ganglia: effect of the dopaminergic denervation on AMPA receptor gene expression in the striatopallidal complex in Parkinson’s disease and rat with 6-OHDA lesion. J Comp Neurol. 1996;368:553–568. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous