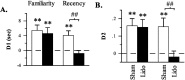Interaction between perirhinal and medial prefrontal cortex is required for temporal order but not recognition memory for objects in rats - PubMed (original) (raw)
Interaction between perirhinal and medial prefrontal cortex is required for temporal order but not recognition memory for objects in rats
Darren K Hannesson et al. J Neurosci. 2004.
Abstract
The present study investigated the roles of the perirhinal cortex, medial prefrontal cortex, and intrahemispheric interactions between them in recognition and temporal order memory for objects. Experiment 1 assessed the effects of bilateral microinfusions of the sodium channel blocker lidocaine into either the anterior perirhinal or medial prefrontal cortex immediately before memory testing in a familiarity discrimination task and a recency discrimination task, both of which involved spontaneous exploration of objects. Inactivation of the perirhinal cortex disrupted performance in both tasks, whereas inactivation of the medial prefrontal cortex disrupted performance in the recency, but not the familiarity, discrimination task. In a second experiment, the importance of intrahemispheric interactions between these structures in temporal order memory were assessed by comparing the effects of unilateral inactivation of either structure alone with those of crossed unilateral inactivation of both structures on the recency discrimination task. Crossed unilateral inactivation of both structures produced a significant impairment, whereas inactivation of either structure alone produced little or no impairment. Collectively, these findings suggest that the perirhinal cortex, but not the medial prefrontal cortex, contributes to retrieval of information necessary for long-term object recognition, whereas both structures, via intrahemispheric interactions between them, contribute to retrieval of information necessary for long-term object temporal order memory. These data are consistent with models in which attributed information is stored in posterior cortical sites and supports lower-order mnemonic functions (e.g., recognition memory) but can also be retrieved and further processed via interactions with the prefrontal cortex to support higher-order mnemonic functions (e.g., temporal order memory).
Figures
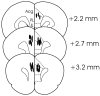
Figure 1.
Location of the injection needles (lines) in rats in the mPFC group included in the behavioral analyses (plates located anterior to bregma and adapted from Paxinos and Watson, 1998). Acg, Anterior cingulate cortex; IL, infralimbic cortex; PL, prelimbic cortex.
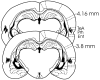
Figure 2.
Location of the injection needles (lines) in rats in the Prh group included in the behavioral analyses (plates located posterior to bregma and adapted from Paxinos and Watson, 1998). Ent, Entorhinal cortex; Prh, perirhinal cortex; TeA, temporal association cortex.
Figure 3.
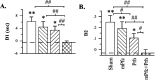
Differences in the amount of exploration directed at novel versus familiar (familiarity discrimination task) or old familiar versus recent familiar (recency discrimination task) objects after sham or lidocaine (Lido) infusions into the mPFC in experiment 1. A, Difference score (D1) calculated as time spent exploring the novel (or old familiar) object less time spent exploring the familiar (or recent familiar) object. B, Weighted difference score (D2) calculated as D1 divided by the time spent exploring both objects. **p < 0.01, one-tailed, relative to chance performance (i.e., 0, indicated by the dashed line); ##p < 0.01, one-tailed, sham versus lidocaine.
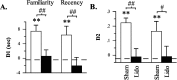
Figure 4.
Differences in the amount of exploration directed at novel versus familiar (familiarity discrimination task) or old familiar versus recent familiar (recency discrimination task) objects after sham or lidocaine (Lido) infusions into the anterior Prh in experiment 1. A, Difference score (D1) calculated as time spent exploring the novel (or old familiar) object less time spent exploring the familiar (or recent familiar) object. B, Weighted difference score (D2) calculated as D1 divided by the time spent exploring both objects. **p < 0.01, one-tailed, relative to chance performance (i.e., 0, indicated by the dashed line); #p < 0.05 and ##p < 0.01, one-tailed, sham versus lidocaine.
Figure 5.
Differences in the amount of exploration directed at old familiar versus recent familiar (recency discrimination task) objects after sham, unilateral, or crossed unilateral infusions into anterior Prh and/or mPFC in experiment 2. A, Difference score (D1) calculated as time spent exploring the old familiar object less time spent exploring the recent familiar object. B, Weighted difference score (D2) calculated as D1 divided by the time spent exploring both objects. *p < 0.05 and **p < 0.01, one-tailed, relative to chance performance (i.e., 0, indicated by the dashed line); #p < 0.05 and ##p < 0.01, one-tailed, sham versus lidocaine.
Similar articles
- What pharmacological interventions indicate concerning the role of the perirhinal cortex in recognition memory.
Brown MW, Barker GR, Aggleton JP, Warburton EC. Brown MW, et al. Neuropsychologia. 2012 Nov;50(13):3122-40. doi: 10.1016/j.neuropsychologia.2012.07.034. Epub 2012 Jul 27. Neuropsychologia. 2012. PMID: 22841990 Free PMC article. Review. - Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex.
Barker GR, Bird F, Alexander V, Warburton EC. Barker GR, et al. J Neurosci. 2007 Mar 14;27(11):2948-57. doi: 10.1523/JNEUROSCI.5289-06.2007. J Neurosci. 2007. PMID: 17360918 Free PMC article. - When is the hippocampus involved in recognition memory?
Barker GR, Warburton EC. Barker GR, et al. J Neurosci. 2011 Jul 20;31(29):10721-31. doi: 10.1523/JNEUROSCI.6413-10.2011. J Neurosci. 2011. PMID: 21775615 Free PMC article. - Altered object exploration but not temporal order memory retrieval in an object recognition test following treatment of rats with the group II metabotropic glutamate receptor agonist LY379268.
Lins BR, Ballendine SA, Howland JG. Lins BR, et al. Neurosci Lett. 2014 Feb 7;560:41-5. doi: 10.1016/j.neulet.2013.12.016. Epub 2013 Dec 17. Neurosci Lett. 2014. PMID: 24361135 Free PMC article. - Findings from animals concerning when interactions between perirhinal cortex, hippocampus and medial prefrontal cortex are necessary for recognition memory.
Warburton EC, Brown MW. Warburton EC, et al. Neuropsychologia. 2010 Jul;48(8):2262-72. doi: 10.1016/j.neuropsychologia.2009.12.022. Epub 2009 Dec 21. Neuropsychologia. 2010. PMID: 20026141 Review.
Cited by
- What pharmacological interventions indicate concerning the role of the perirhinal cortex in recognition memory.
Brown MW, Barker GR, Aggleton JP, Warburton EC. Brown MW, et al. Neuropsychologia. 2012 Nov;50(13):3122-40. doi: 10.1016/j.neuropsychologia.2012.07.034. Epub 2012 Jul 27. Neuropsychologia. 2012. PMID: 22841990 Free PMC article. Review. - Role of CA3 and CA1 subregions of the dorsal hippocampus on temporal processing of objects.
Hoge J, Kesner RP. Hoge J, et al. Neurobiol Learn Mem. 2007 Sep;88(2):225-31. doi: 10.1016/j.nlm.2007.04.013. Epub 2007 Jun 8. Neurobiol Learn Mem. 2007. PMID: 17560815 Free PMC article. - Role of medial prefrontal cortex serotonin 2A receptors in the control of retrieval of recognition memory in rats.
Bekinschtein P, Renner MC, Gonzalez MC, Weisstaub N. Bekinschtein P, et al. J Neurosci. 2013 Oct 2;33(40):15716-25. doi: 10.1523/JNEUROSCI.2087-13.2013. J Neurosci. 2013. PMID: 24089480 Free PMC article. - Mapping parahippocampal systems for recognition and recency memory in the absence of the rat hippocampus.
Kinnavane L, Amin E, Horne M, Aggleton JP. Kinnavane L, et al. Eur J Neurosci. 2014 Dec;40(12):3720-34. doi: 10.1111/ejn.12740. Epub 2014 Sep 29. Eur J Neurosci. 2014. PMID: 25264133 Free PMC article. - Diminished Dentate Gyrus Filtering of Cortical Input Leads to Enhanced Area Ca3 Excitability after Mild Traumatic Brain Injury.
Folweiler KA, Samuel S, Metheny HE, Cohen AS. Folweiler KA, et al. J Neurotrauma. 2018 Jun 1;35(11):1304-1317. doi: 10.1089/neu.2017.5350. Epub 2018 Apr 6. J Neurotrauma. 2018. PMID: 29338620 Free PMC article.
References
- Aggleton JP, Brown MW (1999) Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci 22: 425-444. - PubMed
- Aggleton JP, Keen S, Warburton EC, Bussey TJ (1997) Extensive cytotoxic lesions involving both the rhinal cortices and area TE impair recognition but spare spatial alternation in the rat. Brain Res Bull 43: 279-287. - PubMed
- Bachevalier J, Mishkin M (1986) Visual recognition impairment follows ventromedial but not dorsolateral prefrontal lesions in monkeys. Behav Brain Res 20: 249-261. - PubMed
- Brown MW (1996) Neuronal responses and recognition memory. Semin Neurosci 8: 23-32.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical