Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells - PubMed (original) (raw)
Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells
Yoichi Kosodo et al. EMBO J. 2004.
Abstract
At the onset of neurogenesis in the mammalian central nervous system, neuroepithelial cells switch from symmetric, proliferative to asymmetric, neurogenic divisions. In analogy to the asymmetric division of Drosophila neuroblasts, this switch of mammalian neuroepithelial cells is thought to involve a change in cleavage plane orientation from perpendicular (vertical cleavage) to parallel (horizontal cleavage) relative to the apical surface of the neuroepithelium. Here, we report, using TIS21-GFP knock-in mouse embryos to identify neurogenic neuroepithelial cells, that at the onset as well as advanced stages of neurogenesis the vast majority of neurogenic divisions, like proliferative divisions, show vertical cleavage planes. Remarkably, however, neurogenic divisions of neuroepithelial cells, but not proliferative ones, involve an asymmetric distribution to the daughter cells of the apical plasma membrane, which constitutes only a minute fraction (1-2%) of the entire neuroepithelial cell plasma membrane. Our results support a novel concept for the cell biological basis of asymmetric, neurogenic divisions of neuroepithelial cells in the mammalian central nervous system.
Figures
Figure 1
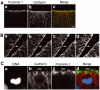
The cadherin ‘hole' as a means of identifying the apical membrane of NE cells. (A) Distinct localization of prominin-1 and cadherin at the apical side of the mouse embryonic neuroepithelium. Double immunolabeling of a frozen section of E9.0 mouse forebrain for prominin-1 (a, c; green) and cadherin (b, c; red). Note that, in the single optical section shown, there is no overlap between the prominin-1 and cadherin staining at the apical side of the neuroepithelium (c), as expected from the specific association of prominin-1 with microvilli of the apical membrane and the concentration of cadherin at the apical-most end of the lateral plasma membrane. Bar in (c)=10 μm. (B) Detection of a cadherin ‘hole' in consecutive optical sections. A frozen section of E10.5 mouse midbrain neuroepithelium was immunostained for cadherin. (a–d) show four consecutive, adjacent 1-μm optical sections obtained by confocal microscopy. The apical membrane, as revealed by the cadherin ‘hole', is indicated by small white bars. Arrows indicate a single cell whose cadherin hole is apparent in only one of the optical sections (c). Bar in (d)=10 μm. (C) The cadherin hole contains the apical membrane-specific protein prominin-1. Triple labeling of a frozen section of an E10.5 mitotic mouse NE cell for DNA (a, d; blue; propidium iodide staining), cadherin (b, d; red) and prominin-1 (c, d; green). White bars indicate the apical membrane of the mitotic NE cell (b–d). Note that, in the single optical section shown, the cadherin staining of the lateral plasma membrane and the prominin-1 staining of the apical membrane are mutually exclusive, and that the cadherin hole is immunostained for prominin-1 (d).
Figure 2
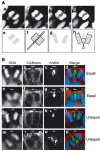
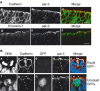
Determination of the cleavage plane and equal versus unequal distribution of the apical membrane during division of NE cells. (A) Determination of the cleavage plane. A frozen section of E9.5 mouse hindbrain neuroepithelium was stained for DNA using propidium iodide. (a–d) Four consecutive, adjacent 1-μm optical sections of a mitotic NE cell in anaphase. The cell shown is a representative example in which, for each optical section, the sister chromatids of the prospective daughter cells appear similar in size and shape, indicating that the planes of scanning of the individual optical sections were perpendicular to the cleavage plane, which in such a case can be deduced accurately (dashed white line in (c)) as illustrated in panels e and f. Only mitotic NE cells fulfilling this criterion (about every fifth cell classified as anaphase or telophase) were included in the subsequent analyses (see Materials and methods). (e–h) Deduction of cleavage plane. The DNA staining as observed in panels c and Ba is shown in (e) and (g) (shaded areas), respectively, and rectangles were designed to fit this staining (f and h, respectively). The orientation of the rectangles relative to each other was deduced (long black lines extending one side of the rectangle). In the example shown in (f), the rectangles are oriented parallel to each other, and the cleavage plane is deduced to be positioned half way in between (dashed red line). In the example shown in (h), the rectangles are oriented at a certain angle to each other, and the cleavage plane is deduced to be positioned such that this angle is halved (dashed red line). (B) Two examples each of an equal (a–d, e–h) and unequal (i–l, m–p) distribution of the apical membrane. (a–d, e–h, i–l and m–p) each show one triple-labeled NE cell in a frozen section from E11.5 mouse midbrain. The cleavage plane (dashed white lines) was deduced from the orientation of the DAPI-stained sister chromatids observed in consecutive optical sections, one of which is shown (a, e, i, m and d, h, l, p; blue), as described in (A). The deduced cleavage plane was corroborated by immunostaining for anillin (c, g, k, o and d, h, l, p; green). The apical membrane, that is, the cadherin hole (white bars), was identified by immunolabeling for cadherin (b, f, j, n and d, h, l, p; red). In the mitotic NE cells shown in the two top rows (a–d and e–h), the apical membrane will be bisected upon cleavage and distributed equally to the daughter cells. In contrast, in the mitotic NE cells shown in the two bottom rows (i–l and m–p), the apical membrane will be bypassed by the cleavage and distributed unequally, that is, to only one daughter cell. Note that, in the mitotic NE cells shown in the second (e–h) and fourth (m–p) rows, the cleavage furrow as revealed by anillin immunostaining has reached the apical (g) and lateral (o) plasma membrane (white arrowheads), respectively, whereas this is not yet the case in the mitotic NE cells shown in the first (a–d) and third (i–l) rows. The asterisk in (n) indicates the cadherin hole of another cell adjacent to the mitotic NE cell shown.
Figure 3
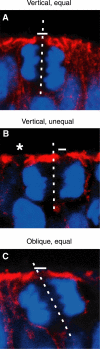
An equal versus unequal distribution of the apical membrane is not necessarily associated with a vertical versus non-vertical cleavage plane of NE cells. Frozen sections of E9.5–10.0 mouse hindbrain neuroepithelium were double-labeled for cadherin (A–C; red) and DNA (A–C; blue; propidium iodide staining). In the single optical section shown, white bars indicate the apical membrane (cadherin hole), and the dashed white lines show the cleavage plane deduced as described in Figure 2A. Note that in (B), the cleavage plane is vertical but the apical membrane distributed unequally, whereas in (C), the cleavage plane is oblique but the apical membrane is distributed equally. The asterisk in (B) indicates the cadherin hole of another cell adjacent to the mitotic NE cell shown.
Figure 4
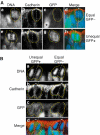
Equal versus unequal distribution of the apical membrane and proliferative versus neurogenic divisions of NE cells in the midbrain of heterozygous E11.5 embryos of _TIS21_-GFP knock-in mice. (A) An example of an equal distribution of the apical membrane in a proliferative division (a–d) and an example of an unequal distribution of the apical membrane in a neurogenic division (e–h). (a–d) and (e–h) each show one triple-labeled mitotic NE cell, outlined by the yellow dots. The cleavage plane (dashed white lines) was deduced from the orientation of the DAPI-stained sister chromatids observed in consecutive optical sections, one of which is shown (DNA; a, e and d, h; blue), as described in Figure 2A. Note that the cleavage plane in both examples is vertical. The apical membrane, that is, the cadherin hole (white bars), was identified by immunolabeling for cadherin (b, f and d, h; red). In the mitotic NE cell in the top row (a–d), the apical membrane will be bisected by the cleavage and distributed equally to the daughter cells, whereas in the mitotic NE cell in the bottom row (e–h), the apical membrane will be bypassed by the cleavage and distributed unequally, that is, to only one daughter cell. The NE cell showing an equal distribution of the apical membrane (a–d) does not express _TIS21_-GFP (GFP−) (c, d; green), indicative of a proliferative division, whereas the NE cell showing an unequal distribution (e–h) does (GFP+) (g, h; green), indicative of a neurogenic division. Note the GFP-expressing cell near the mitotic GFP-negative NE cell in (c) (asterisk). (B) Coexistence in close proximity of proliferating NE cells showing an equal distribution of apical membrane and neurogenic NE cells showing an unequal distribution. Analysis of the two mitotic NE cells shown was performed as described for panels A. The mitotic NE cell on the left distributes its apical membrane unequally, that is, to only one daughter cell, and expresses _TIS21_-GFP (GFP+), indicative of a neurogenic division, whereas the mitotic NE cell on the right distributes its apical membrane equally to the daughter cells and does not express GFP (GFP−), indicative of a proliferative division. Note that for the mitotic NE cell on the right, the cadherin hole is smaller in the optical section shown than in the next optical section (which is not shown).
Figure 5
Neurons arising from asymmetric division of NE cells do not inherit apical membrane and prominin-1. (A) Frozen sections of E11.5 hindbrain (a–e) and E11.5 telencephalon (g–k) of heterozygous _TIS21_-GFP knock-in embryos showing quadruple-labeled dividing NE cells that have almost completed cytokinesis. Single optical sections are shown; DNA, DAPI-stained nuclei (a, e blue, g, k blue). In the NE cell in the top row (a–e), which does not express _TIS21_-GFP (GFP−) (c, e green) and hence undergoes a proliferative division, the cleavage furrow is about to bisect the apical membrane (i.e. the cadherin hole, white bars), as revealed by immunostaining for cadherin (b, e white) and anillin (d, e red) (which at this stage of cytokinesis indicates the midbody (Oegema et al, 2000)), resulting in its distribution to both daughter cells (equal). In contrast, in the NE cell in the bottom row (g–k), which does express _TIS21_-GFP (GFP+) (i, k green) and hence undergoes a neurogenic division, the cleavage furrow is about to bypass the apical membrane (i.e., the cadherin hole, white bars), as revealed by immunostaining for cadherin (h, k white) and anillin (j, k red), resulting in its distribution to only one daughter cell (unequal). (f and l) Cartoons summarizing the imminent completion of cytokinesis by plasma membrane fusion in the cell shown in (e) and (k), respectively; black lines, lateral and cleavage furrow plasma membranes; light blue box, apical membrane; red, anillin; arrowheads, site of cleavage. (B) Frozen section of E10.5 telencephalon of heterozygous _TIS21_-GFP knock-in embryos showing a quadruple-labeled newborn neuron that was identified by immunostaining for βIII-tubulin (βIII-tub, d, e red) and shows GFP fluorescence (c, e green), indicative of its origin from a _TIS21_-GFP-expressing NE cell (Haubensak et al, 2004). A single optical section is shown; DNA, DAPI-stained nuclei (a, e blue). Note the lack of extension of the neuron to the apical surface immunostained for prominin-1 (prom-1, b, e white). (C) Four consecutive optical sections of a newborn neuron (asterisks), identified by immunostaining for βIII-tubulin (red) in a frozen section of E11.5 hindbrain of heterozygous _TIS21_-GFP knock-in embryos. Note the extension of the neuron in the basal direction, but not towards the apical side of the neuroepithelium (top). Blue, DAPI-stained nuclei.
Figure 6
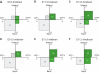
Equal versus unequal distribution of the apical membrane and proliferative versus neurogenic divisions of NE cells at various developmental stages and in various brain regions of heterozygous embryos of _TIS21_-GFP knock-in mice. The distribution of the apical membrane and the expression of _TIS21_-GFP in mitotic NE cells were analyzed for the indicated developmental stages and brain regions as exemplified in Figure 4. For a given developmental stage and brain region, mitotic NE cells were classified into four groups: (i) equal distribution of the apical membrane (equal, ordinate) and _TIS21_-GFP-negative (GFP−, abscissa) (gray, plain), (ii) equal distribution and _TIS21_-GFP-positive (GFP+, abscissa) (green, plain), (iii) unequal distribution of the apical membrane (unequal, ordinate) and _TIS21_-GFP-negative (gray, hatched) and (iv) unequal distribution and _TIS21_-GFP-positive (green, hatched). Cells in each group were expressed as percent of total (sum of the four groups), and the percentage values are indicated by the area of the respective square and the number therein. Numbers of cells were: E9.5 forebrain, _n_=11; E11.5 forebrain, _n_=18; E14.5 forebrain, _n_=15; E10.5 midbrain, _n_=10; E11.5 midbrain, _n_=44 (same data as Table II, shown for reference); and E11.5 hindbrain, _n_=11.
Figure 7
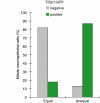
Correlation between the equal versus unequal distribution of the apical membrane and proliferative versus neurogenic divisions of NE cells in heterozygous embryos of _TIS21_-GFP knock-in mice. The distribution of the apical membrane and the expression of _TIS21_-GFP in mitotic NE cells were analyzed as exemplified in Figure 4. For both, mitotic NE cells showing an equal distribution of the apical membrane (left columns, _n_=76) and an unequal distribution (right columns, _n_=39), the percentage of _TIS21_-GFP-negative (gray columns) versus _TIS21_-GFP-positive (green columns) cells was calculated. Data for various developmental stages (E9.5–14.5) and brain regions (forebrain, midbrain, hindbrain) were pooled because the principal observation that the majority of mitotic NE cells showing an equal distribution of the apical membrane are _TIS21_-GFP-negative, that is, proliferating, and the majority of the cells showing an unequal distribution are _TIS21_-GFP-positive, that is, neurogenic, was made irrespective of the developmental stage and brain region analyzed (for details, see Figure 6).
Figure 8
Equal versus unequal distribution of par-3 upon proliferative versus neurogenic divisions of NE cells in the hindbrain of heterozygous E10.5–E12.5 embryos of _TIS21_-GFP knock-in mice. (A) Frozen section double immunofluorescence showing the localization of par-3 (b, c red, e, f red) in comparison with cadherin (a, c green) and prominin-1 (d, f green) at the apical side of the E10.5 (d–f) and E12.5 (a–c) hindbrain neuroepithelium. Note that in the single optical sections shown, the staining for par-3 appears to be more apical than the most apical staining for cadherin, with little overlap (a–c), and is also largely distinct from the staining for prominin-1, which appears to be more apical than the staining for par-3 (d–f). Bars in (c) and (f)=10 μm. (B) An example of an equal distribution of par-3 in a proliferative division (a–e) and an example of an unequal distribution of par-3 in a neurogenic division (f–j). (a–e) and (f–j) Each show one quadruple-labeled mitotic NE cell in a frozen section. The cleavage plane (dashed white lines) and the apical membrane (i.e. the cadherin hole, white bars) were determined from the orientation of the DAPI-stained sister chromatids (DNA, a, e blue, f, j blue) and by immunolabeling for cadherin (b, e white, g, j white) as in Figures 2 and 4. The single optical section shown reveals that in the mitotic NE cell in the top row (a–e), the apical membrane will be bisected by the cleavage and the apical par-3 (d, e red) distributed to both daughter cells (equal). In contrast, in the mitotic NE cell in the bottom row (f–j), the apical membrane will be bypassed by the cleavage and the apical par-3 (i, j red) distributed to only one daughter cell (unequal). The asterisk in (j) indicates the apical par-3 of another cell adjacent to the mitotic NE cell shown. The NE cell showing an equal distribution of the apical par-3 (a–e) does not express _TIS21_-GFP (GFP−) (c, e green), indicative of a proliferative division, whereas the NE cell showing an unequal distribution (f–j) does (GFP+) (h, j green), indicative of a neurogenic division.
Similar articles
- Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis.
Konno D, Shioi G, Shitamukai A, Mori A, Kiyonari H, Miyata T, Matsuzaki F. Konno D, et al. Nat Cell Biol. 2008 Jan;10(1):93-101. doi: 10.1038/ncb1673. Epub 2007 Dec 16. Nat Cell Biol. 2008. PMID: 18084280 - Aspm specifically maintains symmetric proliferative divisions of neuroepithelial cells.
Fish JL, Kosodo Y, Enard W, Pääbo S, Huttner WB. Fish JL, et al. Proc Natl Acad Sci U S A. 2006 Jul 5;103(27):10438-10443. doi: 10.1073/pnas.0604066103. Epub 2006 Jun 23. Proc Natl Acad Sci U S A. 2006. PMID: 16798874 Free PMC article. - Expression of the antiproliferative gene TIS21 at the onset of neurogenesis identifies single neuroepithelial cells that switch from proliferative to neuron-generating division.
Iacopetti P, Michelini M, Stuckmann I, Oback B, Aaku-Saraste E, Huttner WB. Iacopetti P, et al. Proc Natl Acad Sci U S A. 1999 Apr 13;96(8):4639-44. doi: 10.1073/pnas.96.8.4639. Proc Natl Acad Sci U S A. 1999. PMID: 10200315 Free PMC article. - Symmetric versus asymmetric cell division during neurogenesis in the developing vertebrate central nervous system.
Huttner WB, Kosodo Y. Huttner WB, et al. Curr Opin Cell Biol. 2005 Dec;17(6):648-57. doi: 10.1016/j.ceb.2005.10.005. Epub 2005 Oct 21. Curr Opin Cell Biol. 2005. PMID: 16243506 Review. - The cell biology of neurogenesis.
Götz M, Huttner WB. Götz M, et al. Nat Rev Mol Cell Biol. 2005 Oct;6(10):777-88. doi: 10.1038/nrm1739. Nat Rev Mol Cell Biol. 2005. PMID: 16314867 Review.
Cited by
- ASPM and CITK regulate spindle orientation by affecting the dynamics of astral microtubules.
Gai M, Bianchi FT, Vagnoni C, Vernì F, Bonaccorsi S, Pasquero S, Berto GE, Sgrò F, Chiotto AM, Annaratone L, Sapino A, Bergo A, Landsberger N, Bond J, Huttner WB, Di Cunto F. Gai M, et al. EMBO Rep. 2016 Oct;17(10):1396-1409. doi: 10.15252/embr.201541823. Epub 2016 Aug 25. EMBO Rep. 2016. PMID: 27562601 Free PMC article. - A comparison of brain gene expression levels in domesticated and wild animals.
Albert FW, Somel M, Carneiro M, Aximu-Petri A, Halbwax M, Thalmann O, Blanco-Aguiar JA, Plyusnina IZ, Trut L, Villafuerte R, Ferrand N, Kaiser S, Jensen P, Pääbo S. Albert FW, et al. PLoS Genet. 2012 Sep;8(9):e1002962. doi: 10.1371/journal.pgen.1002962. Epub 2012 Sep 27. PLoS Genet. 2012. PMID: 23028369 Free PMC article. - A two-kinesin mechanism controls neurogenesis in the developing brain.
Helmer P, Vallee RB. Helmer P, et al. Commun Biol. 2023 Dec 1;6(1):1219. doi: 10.1038/s42003-023-05604-5. Commun Biol. 2023. PMID: 38040957 Free PMC article. - Transitional Progenitors during Vertebrate Retinogenesis.
Jin K, Xiang M. Jin K, et al. Mol Neurobiol. 2017 Jul;54(5):3565-3576. doi: 10.1007/s12035-016-9899-x. Epub 2016 May 18. Mol Neurobiol. 2017. PMID: 27194297 Review. - Interkinetic nuclear migration and the selection of neurogenic cell divisions during vertebrate retinogenesis.
Baye LM, Link BA. Baye LM, et al. J Neurosci. 2007 Sep 19;27(38):10143-52. doi: 10.1523/JNEUROSCI.2754-07.2007. J Neurosci. 2007. PMID: 17881520 Free PMC article.
References
- Aaku-Saraste E, Hellwig A, Huttner WB (1996) Loss of occludin and functional tight junctions, but not ZO-1, during neural tube closure—remodeling of the neuroepithelium prior to neurogenesis. Dev Biol 180: 664–679 - PubMed
- Cayouette M, Raff M (2002) Asymmetric segregation of Numb: a mechanism for neural specification from Drosophila to mammals. Nat Neurosci 5: 1265–1269 - PubMed
- Cayouette M, Raff M (2003) The orientation of cell division influences cell-fate choice in the developing mammalian retina. Development 130: 2329–2339 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases