Treatment with recombinant interferon-beta reduces inflammation and slows cartilage destruction in the collagen-induced arthritis model of rheumatoid arthritis - PubMed (original) (raw)
Treatment with recombinant interferon-beta reduces inflammation and slows cartilage destruction in the collagen-induced arthritis model of rheumatoid arthritis
Judith van Holten et al. Arthritis Res Ther. 2004.
Abstract
We investigated the therapeutic potential and mechanism of action of IFN-beta protein for the treatment of rheumatoid arthritis (RA). Collagen-induced arthritis was induced in DBA/1 mice. At the first clinical sign of disease, mice were given daily injections of recombinant mouse IFN-beta or saline for 7 days. Disease progression was monitored by visual clinical scoring and measurement of paw swelling. Inflammation and joint destruction were assessed histologically 8 days after the onset of arthritis. Proteoglycan depletion was determined by safranin O staining. Expression of cytokines, receptor activator of NF-kappaB ligand, and c-Fos was evaluated immunohistochemically. The IL-1-induced expression of IL-6, IL-8, and granulocyte/macrophage-colony-stimulating factor (GM-CSF) was studied by ELISA in supernatant of RA and osteoarthritis fibroblast-like synoviocytes incubated with IFN-beta. We also examined the effect of IFN-beta on NF-kappaB activity. IFN-beta, at 0.25 microg/injection and higher, significantly reduced disease severity in two experiments, each using 8-10 mice per treatment group. IFN-beta-treated animals displayed significantly less cartilage and bone destruction than controls, paralleled by a decreased number of positive cells of two gene products required for osteoclastogenesis, receptor activator of NF-kappaB ligand and c-Fos. Tumor necrosis factor alpha and IL-6 expression were significantly reduced, while IL-10 production was increased after IFN-beta treatment. IFN-beta reduced expression of IL-6, IL-8, and GM-CSF in RA and osteoarthritis fibroblast-like synoviocytes, correlating with reduced NF-kappaB activity. The data support the view that IFN-beta is a potential therapy for RA that might help to diminish both joint inflammation and destruction by cytokine modulation.
Figures
Figure 1
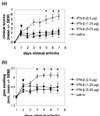
Effect of systemically delivered IFN-β at the onset of arthritis in mice with collagen-induced arthritis. (a) Clinical score (mean ±
SEM
) was assessed on a scale of 0 to 3.5 (as described in Materials and methods), and (b) hind paw swelling (mean ±
SEM
) of the first arthritic paw was monitored during the course of disease using calipers. On day 7 after the start of the treatment, differences between all treatment groups and the control group were statistically significant (P = 0.005) (Kruskal–Wallis test). Arrows mark the first day of treatment. *Statistically significant difference.
SEM
, standard error of the mean.
Figure 2
Representative histologic staining (hematoxylin and eosin; ×100) of the ankle joints in mice with collagen-induced arthritis (CIA). At day 7 after the start of treatment with IFN-β, mice were sacrificed and subjected to histopathological examination. (a) In the control CIA mice treated with saline, massive cellular infiltration and erosion of bone were observed in the ankle joint. In CIA mice treated with the highest dose of IFN-β (2.5 μg per injection per mouse), limited hyperplasia of the intimal lining layer and cell infiltration of the synovial sublining were detected and a decrease in bone erosions was observed. (b) Infiltration of inflammatory cells of the joints was scored from 0 to 4 in a blinded manner as described in Materials and methods. A significant reduction in inflammatory cells was observed for the treatment groups treated with 2.5 μg and 1.25 μg IFN-β in comparison with controls (P = 0.04). *Statistically significant difference.
Figure 3
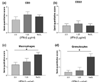
T-cell, B-cell, macrophage, and granulocyte expression in the synovium of mice with collagen-induced arthritis, after 7 days of IFN-β therapy, detected by immunohistochemistry. Sections were scored for CD3 and CD22 as described in Materials and methods. Macrophages and granulocytes were evaluated by morphology. (a) CD3 expression. (b) CD22 expression. (c) Macrophage expression. (d) Granulocyte expression. No significant differences between treated animals and controls were observed for CD3 and CD22 expression (P = 0.4 for both). Statistically significant differences were observed for macrophage and granulocyte expression between IFN-β-treated mice and saline-treated mice (P = 0.04 and 0.009, respectively). *Statistically significant difference.
Figure 4
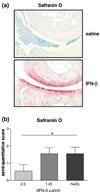
Representative histologic staining with safranin O–fast green (×100) of the ankle joint of mice with collagen-induced arthritis (CIA) mice after daily IFN-β therapy for 7 days. (a) (Upper panel) In CIA mice treated with saline as controls, hardly any safranin O staining was observed in the ankle joints. (Lower panel) In CIA mice treated daily with 2.5 μg IFN-β, significantly less loss of safranin O staining was observed, indicating inhibition of cartilage breakdown. (b) Histologic analysis of cartilage in CIA in mice after 7 days of IFN-β therapy. Hind paw sections were stained with safranin O–fast green, which stains the cartilage proteoglycans. Sections were scored in a blinded manner on a 4-point scale as described in Materials and methods. Significantly less loss of safranin O staining was observed in the animals treated with IFN 2.5 μg than in controls (P = 0.03), indicating inhibition of cartilage destruction. *Statistically significant difference.
Figure 5
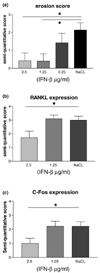
Histologic analysis of bone erosions in mice with collagen-induced arthritis (CIA) after treatment with IFN-β. Mice were treated with the indicated concentrations of IFN-β for 7 days starting when the first clinical signs of arthritis were observed. Hind paws were taken for histology. (a) Bone erosions were scored in a blinded manner on a scale of 0 to 4 as described in Materials and methods. A significant reduction in bone erosions was observed in the animals treated with 2.5 μg and 1.25 μg IFN-β per injection/mouse in comparison with controls (P = 0.02); results are means ±
SEM
. Immunohistochemical analysis of the number of cells positive for RANKL (receptor activator of NF-κB ligand) and c-Fos after 7 days of IFN-β treatment in CIA mice. Sections were scored on a 4-point scale. (b) RANKL staining; a decrease of RANKL-positive cells was observed in the group of animals treated with 2.5 μg IFN-β, although not statistically significant (P = 0.07). (c) c-Fos staining; the number of c-Fos-positive cells was significantly reduced in the mice treated with daily 2.5 μg per injection IFN-β (P = 0.04). *Statistically significant difference.
SEM
, standard error of the mean.
Figure 6
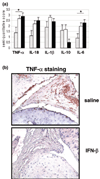
(a) Cytokine expression measured by immunohistochemistry after 7 days of systemic IFN-β treatment in mice with collagen-induced arthritis (CIA). Tumor necrosis factor (TNF)-α, IL-18, IL-1β, IL-10, and IL-6 were scored (mean ±
SEM
) in a blinded manner on a scale of 0 to 4 as described in Materials and methods. A statistically significant reduction of the proinflammatory cytokines TNF-α and IL-6 (P = 0.03 and 0.02, respectively) was observed in the mice treated with 2.5 μg IFN-β in comparison with controls. The expression of IL-18 and IL-1β was reduced in the animals treated with the highest dose of IFN-β, and the expression of IL-10 was higher than in controls. These differences did not reach statistical significance. No clear-cut differences were observed in the animals treated with 1.25 μg IFN-β in comparison with controls. Bars represent mice treated with 2.5 μg IFN-β (grey), 1.25 μg IFN-β (white) or saline (black). (b) Representative immunohistologic staining showing TNF-α expression in the ankle joints in CIA mice after 7 days of IFN-β treatment, assessed by immunohistochemistry. (Upper panel) TNF-α expression in CIA mice treated with saline; abundant expression of TNF-α was observed in the ankle joint. (Lower panel) TNF-α expression in CIA mice treated with 2.5 μg IFN-β; in the group treated with the highest dose of IFN-β, only a few positive cells for TNF-α were observed. *Statistically significant difference.
SEM
, standard error of the mean.
Figure 7
IL-6, IL-8, and GM-CSF (granulocyte/macrophage-colony-stimulating factor) production in synoviocytes from patients with rheumatoid arthritis (RA) and osteoarthritis (OA) after incubation with increasing concentrations of IFN-β, measured in supernatant of fibroblast-like synoviocytes using enzyme-linked immunosorbent assay. Decreased production of (a) IL-6, (b) IL-8, and (b) GM-CSF in RA and OA fibroblast-like synoviocytes after 48 hours' incubation with increasing concentrations of IFN-β.
Figure 8
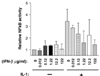
Relative NF-κB activity measured in NF-κB-transfected rheumatoid arthritis synoviocytes after incubation with increasing concentration of IFN-β. Unactivated fibroblast-like synoviocytes showed low NF-κB activity. IL-1β-induced NF-κB activity revealed a trend towards inhibition after incubation with IFN-β in increasing concentrations.
Similar articles
- Protection against cartilage and bone destruction by systemic interleukin-4 treatment in established murine type II collagen-induced arthritis.
Joosten LA, Lubberts E, Helsen MM, Saxne T, Coenen-de Roo CJ, Heinegård D, van den Berg WB. Joosten LA, et al. Arthritis Res. 1999;1(1):81-91. doi: 10.1186/ar14. Epub 1999 Oct 26. Arthritis Res. 1999. PMID: 11056663 Free PMC article. - Bone- and cartilage-protective effects of a monoclonal antibody against colony-stimulating factor 1 receptor in experimental arthritis.
Toh ML, Bonnefoy JY, Accart N, Cochin S, Pohle S, Haegel H, De Meyer M, Zemmour C, Preville X, Guillen C, Thioudellet C, Ancian P, Lux A, Sehnert B, Nimmerjahn F, Voll RE, Schett G. Toh ML, et al. Arthritis Rheumatol. 2014 Nov;66(11):2989-3000. doi: 10.1002/art.38624. Arthritis Rheumatol. 2014. PMID: 24623505 - Inhibitory effects of niclosamide on inflammation and migration of fibroblast-like synoviocytes from patients with rheumatoid arthritis.
Liang L, Huang M, Xiao Y, Zen S, Lao M, Zou Y, Shi M, Yang X, Xu H. Liang L, et al. Inflamm Res. 2015 Apr;64(3-4):225-33. doi: 10.1007/s00011-015-0801-5. Epub 2015 Feb 24. Inflamm Res. 2015. PMID: 25708600 - IFN-beta in rheumatoid arthritis.
Tak PP. Tak PP. Front Biosci. 2004 Sep 1;9:3242-7. doi: 10.2741/1475. Front Biosci. 2004. PMID: 15353352 Review. - Role of PGE2 and EP receptors in the pathogenesis of rheumatoid arthritis and as a novel therapeutic strategy.
Akaogi J, Nozaki T, Satoh M, Yamada H. Akaogi J, et al. Endocr Metab Immune Disord Drug Targets. 2006 Dec;6(4):383-94. doi: 10.2174/187153006779025711. Endocr Metab Immune Disord Drug Targets. 2006. PMID: 17214584 Review.
Cited by
- Osteoimmunology and the influence of pro-inflammatory cytokines on osteoclasts.
Zupan J, Jeras M, Marc J. Zupan J, et al. Biochem Med (Zagreb). 2013;23(1):43-63. doi: 10.11613/bm.2013.007. Biochem Med (Zagreb). 2013. PMID: 23457765 Free PMC article. Review. - Localized delivery of interferon-β by Lactobacillus exacerbates experimental colitis.
McFarland AP, Savan R, Wagage S, Addison A, Ramakrishnan K, Karwan M, Duong T, Young HA. McFarland AP, et al. PLoS One. 2011 Feb 18;6(2):e16967. doi: 10.1371/journal.pone.0016967. PLoS One. 2011. PMID: 21365015 Free PMC article. - Optimization of current and future therapy for autoimmune diseases.
Steinman L, Merrill JT, McInnes IB, Peakman M. Steinman L, et al. Nat Med. 2012 Jan 6;18(1):59-65. doi: 10.1038/nm.2625. Nat Med. 2012. PMID: 22227674 No abstract available. - Emerging concepts of type I interferons in SLE pathogenesis and therapy.
Psarras A, Wittmann M, Vital EM. Psarras A, et al. Nat Rev Rheumatol. 2022 Oct;18(10):575-590. doi: 10.1038/s41584-022-00826-z. Epub 2022 Sep 12. Nat Rev Rheumatol. 2022. PMID: 36097207 Review. - Local delivery of beta interferon using an adeno-associated virus type 5 effectively inhibits adjuvant arthritis in rats.
Adriaansen J, Fallaux FJ, de Cortie CJ, Vervoordeldonk MJ, Tak PP. Adriaansen J, et al. J Gen Virol. 2007 Jun;88(Pt 6):1717-1721. doi: 10.1099/vir.0.82603-0. J Gen Virol. 2007. PMID: 17485531 Free PMC article.
References
- De ME, De Maeyer-Guignard J. Type I interferons. Int Rev Immunol. 1998;17:53–73. - PubMed
- Rep MH, Hintzen RQ, Polman CH, van Lier RA. Recombinant interferon-beta blocks proliferation but enhances interleukin-10 secretion by activated human T-cells. J Neuroimmunol. 1996;67:111–118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical