Autophosphorylation of JAK2 on tyrosines 221 and 570 regulates its activity - PubMed (original) (raw)
Autophosphorylation of JAK2 on tyrosines 221 and 570 regulates its activity
Lawrence S Argetsinger et al. Mol Cell Biol. 2004 Jun.
Abstract
The tyrosine kinase JAK2 is a key signaling protein for at least 20 receptors in the cytokine/hematopoietin receptor superfamily and is a component of signaling by insulin receptor and several G-protein-coupled receptors. However, there is only limited knowledge of the physical structure of JAK2 or which of the 49 tyrosines in JAK2 are autophosphorylated. In this study, mass spectrometry and two-dimensional peptide mapping were used to determine that tyrosines 221, 570, and 1007 in JAK2 are autophosphorylated. Phosphorylation of tyrosine 570 is particularly robust. In response to growth hormone, JAK2 was rapidly and transiently phosphorylated at tyrosines 221 and 570, returning to basal levels by 60 min. Analysis of the sequences surrounding tyrosines 221 and 570 in JAK2 and tyrosines in other proteins that are phosphorylated in response to ligands that activate JAK2 suggests that the YXX[L/I/V] motif is one of the motifs recognized by JAK2. Experiments using JAK2 with tyrosines 221 and 570 mutated to phenylalanine suggest that tyrosines 221 and 570 in JAK2 may serve as regulatory sites in JAK2, with phosphorylation of tyrosine 221 increasing kinase activity and phosphorylation of tyrosine 570 decreasing kinase activity and thereby contributing to rapid termination of ligand activation of JAK2.
Figures
FIG. 1.
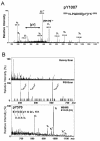
JAK2 is autophosphorylated on tyrosines 221 and 1007. Phosphorylation at Y221 (A) and Y1007 (B) of JAK2 was identified using μLC/MS/MS. MS/MS spectra were obtained on a Finnigan LCQ quadripole ion trap mass spectrometer. MS/MS spectra derived from the sequences of doubly charged tryptic peptides IQDYpHILTR (upper spectrum) and VLPQDKEYpYK (lower spectrum) are shown. Amino acid sequences from phosphopeptides could be identified from the b or Y ion fragment peaks. Phosphorylated tyrosines (Y221 and Y1007) are indicated by Y (80), corresponding to an 80-Da increment of the molecular mass of the tyrosine residue.
FIG. 2.
JAK2 is autophosphorylated on tyrosines 570 and 1007. (A) Nanoelectrospray quadrupole time-of-flight MS/MS spectrum of the ion corresponding to the phosphorylated peptide T1000-1009 (VLPQDKEpYYK), revealing the phosphotyrosine residue at position 1007. Y-ion series corresponding to C-terminal peptide ion fragments and a- and b-ions corresponding to N-terminal fragment ions are indicated. (B) The MS survey scan of the OLIGO R3 20% methanol fraction is shown in the upper panel. The middle panel shows the phosphotyrosine-specific PSI scan (m/z 216.043) of the same fraction. Three ion signals at m/z 409, 461, and 613 are observable. Subsequent MS/MS experiments revealed that the tyrosine-phosphorylated precursors correspond to the doubly and triply charged tryptic peptide T566-575 (EVGDpYGQLHK) and the triply charged tryptic peptide T565-575 (REVGDpYGQLHK). The bottom panel shows the MS/MS spectrum of the doubly charged tryptic peptide T566-575 (EVGDpYGQLHK) at m/z 635.2. The m/z range above the m/z value of the precursor is shown.
FIG. 3.
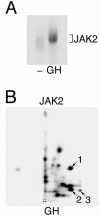
JAK2 is autophosphorylated on tyrosines 221 and 570 in vitro. (A) 293T cells expressing the cDNA for JAK2, JAK2 Y221F, or JAK2 Y570F were lysed, and JAK2 was immunoprecipitated using α-JAK2. The JAK2 was immobilized and incubated in the presence of [γ-32P]ATP at 30°C for 30 min. The migration of JAK2 is indicated. (B) In a parallel experiment, 293T cells expressing the cDNA for JAK2, JAK2 Y221F, or JAK2 Y570F were lysed, and JAK2 was immunoprecipitated using α-JAK2. Lysates and the immunoprecipitated JAK2 were blotted with α-JAK2. The migration of JAK2 is indicated. (C to F) The 32P-labeled JAK2 shown in panel A was cut from the nitrocellulose and subjected to 2-D peptide mapping, with the TLE step performed at pH 1.9 (8). Spot 1 disappears when tyrosine 221 is mutated to phenylalanine. Spots 2 and 3 disappear when tyrosine 570 is mutated to phenylalanine. The origin (+) is indicated. (G) 32P-labeled peptides corresponding to spots 1 and 2 were scraped from the cellulose plate used for panel C and subjected to phospho-amino acid analysis. Full-length JAK2 (starting material) was also subjected to phospho-amino acid analysis. The migration of phosphotyrosine (ptyr), phosphothreonine (pthr), and phosphoserine (pser) are indicated.
FIG. 4.
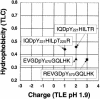
Theoretical migration of the peptides containing tyrosines 221 and 570 on 2-D peptide maps. The theoretical migrations of peptides containing tyrosines 221 (IQDpYHILTR and IQDpYHILpTR) and 570 (EVGDpYGQLHK and REVGDpYGQLHK) in 2-D peptide maps were calculated based on the parameters of Boyle et al. (8) for TLE at pH 1.9 and thin layer chromatography in phospho-chromatography buffer.
FIG. 5.
JAK2 is autophosphorylated on tyrosines 221 and 570 in vivo. 293T cells expressing the cDNA for SH2-Bβ and JAK2 (A), JAK2 Y221F (B), or JAK2 Y570F (C) were incubated for 4 h in the presence of [32P]orthophosphate. Cells were lysed, and JAK2 was immunoprecipitated using α-JAK2. [32P]JAK2 was isolated and subjected to 2-D peptide mapping, with the TLE step performed at pH 3.5 (8).
FIG. 6.
JAK2 activated in response to GH is phosphorylated on tyrosines 221 and 570. (A) 3T3-F442A cells were incubated in the absence or presence of 23 ng of GH/ml for 15 min. The cells were lysed, and JAK2 was immunoprecipitated using α-JAK2. The JAK2 was immobilized and incubated in the presence of [γ-32P]ATP at 30°C for 30 min. The JAK2 was resolved by SDS-PAGE, transferred to nitrocellulose, and visualized by autoradiography. (B) 32P-labeled JAK2 was cut from the nitrocellulose and subjected to 2-D peptide mapping, with the TLE step performed at pH 1.9 (8). The origin (+) and spots whose migration is similar to spots 1, 2, and 3 in Fig. 3 are indicated. Modified from Fig. 3 of reference .
FIG. 7.
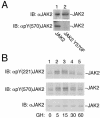
JAK2 is transiently phosphorylated on tyrosines 221 and 570 in response to GH. (A) 293T cells expressing the cDNA for JAK2 or JAK2 Y570F were lysed, and the lysates were blotted (IB) with α-JAK2 or α-pY(570) JAK2. The migration of JAK2 is indicated. (B) 3T3-F442A cells treated with vehicle or 500 ng of GH/ml for the indicated times were lysed, and the lysates were blotted (IB) with α-JAK2, α-pY(221) JAK2, or α-pY(570) JAK2. The migration of JAK2 is indicated.
FIG. 8.
Mutation of JAK2 at tyrosines 221 and 570 affects the ability of JAK2 to autophosphorylate. 293T cells expressing the cDNA for JAK2, JAK2 Y221F, and JAK2 Y570F were lysed. Proteins were immunoprecipitated using α-JAK2 and resolved on SDS-PAGE gels. Lysates were blotted (IB) with α-JAK2 (upper panel) or α-pY(1007, 1008) JAK2 (second panel). The immunoprecipitated JAK2 was blotted with antiphosphotyrosine (αPY; third panel) or α-pY(1007, 1008) JAK2 (bottom panel). The migration of JAK2 is indicated.
FIG. 9.
Tyrosine 570 is the predominant site of phosphorylation in JAK2 Y1007F in vitro. JAK2 was isolated from 293T cells expressing the cDNA for SH2-Bβ and either JAK2 or JAK2 Y1007F, phosphorylated in vitro, and subjected to 2-D peptide mapping as described in the legend for Fig. 3. The TLE step was performed at pH 1.9. The origin (+) is marked. A light and a darker exposure of each plate are shown.
FIG. 10.
Mutation of JAK2 at tyrosines 221 and 570 affects the ability of JAK2 to phosphorylate Stat5b. 293T cells expressing the cDNA for Stat5b and either JAK2, JAK2 Y221F, JAK2 Y570F, JAK2 Y1007F, or vector were lysed, resolved on SDS-PAGE gels, and blotted (IB) with α-Stat5b, α-phosphoStat5b, α-pY(1007,1008) JAK2, antiphosphotyrosine (α-PY), or α-JAK2, as indicated. The migrations of Stat5b and JAK2 are indicated.
Similar articles
- Tyrosines 868, 966, and 972 in the kinase domain of JAK2 are autophosphorylated and required for maximal JAK2 kinase activity.
Argetsinger LS, Stuckey JA, Robertson SA, Koleva RI, Cline JM, Marto JA, Myers MG Jr, Carter-Su C. Argetsinger LS, et al. Mol Endocrinol. 2010 May;24(5):1062-76. doi: 10.1210/me.2009-0355. Epub 2010 Mar 19. Mol Endocrinol. 2010. PMID: 20304997 Free PMC article. - Phosphorylation of JAK2 at serine 523: a negative regulator of JAK2 that is stimulated by growth hormone and epidermal growth factor.
Mazurkiewicz-Munoz AM, Argetsinger LS, Kouadio JL, Stensballe A, Jensen ON, Cline JM, Carter-Su C. Mazurkiewicz-Munoz AM, et al. Mol Cell Biol. 2006 Jun;26(11):4052-62. doi: 10.1128/MCB.01591-05. Mol Cell Biol. 2006. PMID: 16705159 Free PMC article. - Tyrosine phosphorylation of the EGF receptor by the kinase Jak2 is induced by growth hormone.
Yamauchi T, Ueki K, Tobe K, Tamemoto H, Sekine N, Wada M, Honjo M, Takahashi M, Takahashi T, Hirai H, Tushima T, Akanuma Y, Fujita T, Komuro I, Yazaki Y, Kadowaki T. Yamauchi T, et al. Nature. 1997 Nov 6;390(6655):91-6. doi: 10.1038/36369. Nature. 1997. PMID: 9363897 - Tyrosine 813 is a site of JAK2 autophosphorylation critical for activation of JAK2 by SH2-B beta.
Kurzer JH, Argetsinger LS, Zhou YJ, Kouadio JL, O'Shea JJ, Carter-Su C. Kurzer JH, et al. Mol Cell Biol. 2004 May;24(10):4557-70. doi: 10.1128/MCB.24.10.4557-4570.2004. Mol Cell Biol. 2004. PMID: 15121872 Free PMC article. - The Croonian Lecture 1997. The phosphorylation of proteins on tyrosine: its role in cell growth and disease.
Hunter T. Hunter T. Philos Trans R Soc Lond B Biol Sci. 1998 Apr 29;353(1368):583-605. doi: 10.1098/rstb.1998.0228. Philos Trans R Soc Lond B Biol Sci. 1998. PMID: 9602534 Free PMC article. Review.
Cited by
- JAK2S523L, a novel gain-of-function mutation in a critical autoregulatory residue in JAK2V617F- MPNs.
Pastore F, Krishnan A, Hammarén HM, Silvennoinen O, Yan B, Levine RL. Pastore F, et al. Blood Adv. 2020 Sep 22;4(18):4554-4559. doi: 10.1182/bloodadvances.2019001283. Blood Adv. 2020. PMID: 32956452 Free PMC article. - Recent advances in growth hormone signaling.
Lanning NJ, Carter-Su C. Lanning NJ, et al. Rev Endocr Metab Disord. 2006 Dec;7(4):225-35. doi: 10.1007/s11154-007-9025-5. Rev Endocr Metab Disord. 2006. PMID: 17308965 Review. - Two independent histidines, one in human prolactin and one in its receptor, are critical for pH-dependent receptor recognition and activation.
Kulkarni MV, Tettamanzi MC, Murphy JW, Keeler C, Myszka DG, Chayen NE, Lolis EJ, Hodsdon ME. Kulkarni MV, et al. J Biol Chem. 2010 Dec 3;285(49):38524-33. doi: 10.1074/jbc.M110.172072. Epub 2010 Sep 30. J Biol Chem. 2010. PMID: 20889499 Free PMC article. - Jak2 FERM domain interaction with the erythropoietin receptor regulates Jak2 kinase activity.
Funakoshi-Tago M, Pelletier S, Moritake H, Parganas E, Ihle JN. Funakoshi-Tago M, et al. Mol Cell Biol. 2008 Mar;28(5):1792-801. doi: 10.1128/MCB.01447-07. Epub 2007 Dec 26. Mol Cell Biol. 2008. PMID: 18160720 Free PMC article. - 2022 Cannon lecture: an ode to signal transduction: how the growth hormone pathway revealed insight into height, malignancy, and obesity.
Carter-Su C, Argetsinger LS, Svezhova N. Carter-Su C, et al. Am J Physiol Endocrinol Metab. 2023 Nov 1;325(5):E425-E437. doi: 10.1152/ajpendo.00265.2023. Epub 2023 Sep 6. Am J Physiol Endocrinol Metab. 2023. PMID: 37672248 Free PMC article. Review.
References
- Ali, S., and S. Ali. 1998. Prolactin receptor regulates Stat5 tyrosine phosphorylation and nuclear translocation by two separate pathways. J. Biol. Chem. 273:7709-7716. - PubMed
- Arai, A., E. Kanda, Y. Nosaka, N. Miyasaka, and O. Miura. 2001. CrkL is recruited through its SH2 domain to the erythropoietin receptor and plays a role in Lyn-mediated receptor signaling. J. Biol. Chem. 276:33282-33290. - PubMed
- Argetsinger, L. S., G. S. Campbell, X. Yang, B. A. Witthuhn, O. Silvennoinen, J. N. Ihle, and C. Carter-Su. 1993. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 74:237-244. - PubMed
- Argetsinger, L. S., and C. Carter-Su. 1996. Mechanism of signaling by growth hormone receptor. Physiol. Rev. 76:1089-1107. - PubMed
- Banks, A. S., S. M. Davis, S. H. Bates, and M. G. Myers, Jr. 2000. Activation of downstream signals by the long form of the leptin receptor. J. Biol. Chem. 275:14563-14572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK34171/DK/NIDDK NIH HHS/United States
- P60-DK20572/DK/NIDDK NIH HHS/United States
- P30 CA046592/CA/NCI NIH HHS/United States
- R37 DK034171/DK/NIDDK NIH HHS/United States
- P30 CA46592/CA/NCI NIH HHS/United States
- P60-AR20557/AR/NIAMS NIH HHS/United States
- P60 DK020572/DK/NIDDK NIH HHS/United States
- R01 DK034171/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous