Molecular changes in neurons in multiple sclerosis: altered axonal expression of Nav1.2 and Nav1.6 sodium channels and Na+/Ca2+ exchanger - PubMed (original) (raw)
Molecular changes in neurons in multiple sclerosis: altered axonal expression of Nav1.2 and Nav1.6 sodium channels and Na+/Ca2+ exchanger
Matthew J Craner et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Although voltage-gated sodium channels are known to be deployed along experimentally demyelinated axons, the molecular identities of the sodium channels expressed along axons in human demyelinating diseases such as multiple sclerosis (MS) have not been determined. Here we demonstrate changes in the expression of sodium channels in demyelinated axons in MS, with Nav1.6 confined to nodes of Ranvier in controls but with diffuse distribution of Nav1.2 and Nav1.6 along extensive regions of demyelinated axons within acute MS plaques. Using triple-labeled fluorescent immunocytochemistry, we also show that Nav1.6, which is known to produce a persistent sodium current, and the Na+/Ca2+ exchanger, which can be driven by persistent sodium current to import damaging levels of calcium into axons, are colocalized with beta-amyloid precursor protein, a marker of axonal injury, in acute MS lesions. Our results demonstrate the molecular identities of the sodium channels expressed along demyelinated and degenerating axons in MS and suggest that coexpression of Nav1.6 and Na+/Ca2+ exchanger is associated with axonal degeneration in MS.
Figures
Fig. 1.
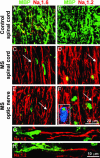
Nav1.6 and Nav1.2 sodium channels are expressed along extensive regions of demyelinated axons in MS. Shown are representative images demonstrating sections of white matter from spinal cord (A_–_D) and optic nerve (E and F) immunostained for MBP (green) as a marker of myelination and for sodium channels Nav1.6 (A, C, and E; red) and Nav1.2 (B, D, and F; red). Control spinal cord white matter (A and B) demonstrates robust MBP immunostaining consistent with myelinated axons and does not display axonal profiles with diffuse (>10 μm) sodium-channel immunostaining. Small foci of Nav1.6 immunostaining (A; yellow arrows) are present, consistent with the focal distribution of Nav1.6 at nodes of Ranvier in control spinal cord white matter, whereas Nav1.2 immunostaining is absent (B). Within acute MS lesions from spinal cord (C and D) and optic nerve (E and F), there is significant demyelination as evident by marked attenuation of MBP immunostaining (residual foci of MBP immunostaining represent intracellular MBP products within macrophages, identified with Ricinus communis agglutinin 1 labeling; blue, F Inset). Multiple axonal profiles within these lesions display diffuse sodium-channel immunostaining (extending in many axons for >20 μm; C, D, E, and F, white arrows) for Nav1.6 (C and E) and Nav1.2 (D and F). (G and H) Shown is the edge of active spinal cord plaques in MS and diffuse sodium-channel immunostaining for Nav1.6 (G; red) and Nav1.2 (H; red) along regions of axons where MBP immunostaining (green) is absent or markedly attenuated.
Fig. 2.
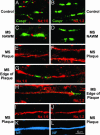
Nav1.6 and Nav1.2 immunostaining in human control CNS and in MS. Shown are representative digital images of sections of postmortem spinal cord white matter from control (A and B) and MS (C_–_L) patients, immunostained to show Nav1.6 (red), Nav1.2 (red), Caspr (green), and neurofilaments (blue). In control white matter (A) and in normal-appearing white matter (NAWM) in MS tissue (C), Nav1.6 is localized at nodes of Ranvier and is bounded by Caspr without appreciable overlap, whereas Nav1.2 is not detectable (B and D). Within MS plaques, linear axonal profiles with continuous Nav1.6 (E) and Nav1.2 (F) immunostaining are present. In some instances an extensive zone of Nav1.6 (G) or Nav1.2 (H) immunostaining is bounded by Caspr, without overlap. Colocalization of Nav1.6 (I) and Nav1.2 (J) with neurofilament immunostaining (SMI 31/32; K and L; blue) further establishes the identity of these profiles as axons.
Fig. 3.
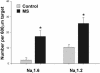
Increased number of axons with extensive Nav1.6 and Nav1.2 immunostaining in MS spinal cord white matter. This histogram demonstrates a significant increase in the number of axons displaying diffuse sodium-channel immunostaining extending >10 μm along the fiber axis in MS spinal cord lesions. *, P < 0.05 compared with controls.
Fig. 4.
(A) Increased β-APP expression in acute MS lesions. This histogram illustrates a significant increase in the number of axonal profiles that are β-APP-positive in MS. *, P < 0.001 compared with controls. (B) NCX and Nav1.6 are coexpressed in β-APP-positive axons in MS. Triple immunolabeling was used to determine the proportion of β-APP-positive axons, and β-APP-negative axons, that coexpress NCX and Nav1.6, or NCX and Nav1.2, over extensive regions. The proportion of axons that coexpress Nav1.6 and NCX is significantly higher in β-APP-positive axons than in β-APP-negative axons. *, P < 0.005.
Fig. 5.
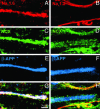
β-APP-positive spinal cord axons coexpress NCX and Nav1.6 over extensive regions in acute MS lesions. Digital images demonstrate axons in MS spinal cord white matter immunostained for β-APP (E and F; blue), sodium channel Nav1.6 (A; red) or Nav1.2 (B; red), and NCX (C and D; green). G and H correspond to merged images (white). A, C, E, and G show coexpression of Nav1.6 and NCX within axons displaying β-APP, a marker of axonal injury. In contrast, B, D, F, and H demonstrate NCX-immunopositive staining but an absence of Nav1.2 immunostaining within β-APP-positive axons, and coexpression of NCX and Nav1.2 within β-APP-negative axons.
Fig. 6.
Proposed mechanism of axonal injury by means of coexpression of Nav1.6 and NCX. The model suggests that Nav1.6 sodium channels are up-regulated (1) and expressed along some demyelinated axons, where they produce persistent sodium current (2). The persistent sodium current can drive reverse sodium/calcium exchange (3) and accumulation of intraaxonal calcium (4), triggering injurious secondary cascades and axonal injury.
Similar articles
- Sodium channel expression within chronic multiple sclerosis plaques.
Black JA, Newcombe J, Trapp BD, Waxman SG. Black JA, et al. J Neuropathol Exp Neurol. 2007 Sep;66(9):828-37. doi: 10.1097/nen.0b013e3181462841. J Neuropathol Exp Neurol. 2007. PMID: 17805013 - Co-localization of sodium channel Nav1.6 and the sodium-calcium exchanger at sites of axonal injury in the spinal cord in EAE.
Craner MJ, Hains BC, Lo AC, Black JA, Waxman SG. Craner MJ, et al. Brain. 2004 Feb;127(Pt 2):294-303. doi: 10.1093/brain/awh032. Epub 2003 Dec 8. Brain. 2004. PMID: 14662515 - Axonal expression of sodium channels and neuropathology of the plaques in multiple sclerosis.
Bouafia A, Golmard JL, Thuries V, Sazdovitch V, Hauw JJ, Fontaine B, Seilhean D. Bouafia A, et al. Neuropathol Appl Neurobiol. 2014 Aug;40(5):579-90. doi: 10.1111/nan.12059. Neuropathol Appl Neurobiol. 2014. PMID: 23659577 - Sodium channels as molecular targets in multiple sclerosis.
Waxman SG. Waxman SG. J Rehabil Res Dev. 2002 Mar-Apr;39(2):233-42. J Rehabil Res Dev. 2002. PMID: 12051467 Review. - Demyelination in spinal cord injury and multiple sclerosis: what can we do to enhance functional recovery?
Waxman SG. Waxman SG. J Neurotrauma. 1992 Mar;9 Suppl 1:S105-17. J Neurotrauma. 1992. PMID: 1588601 Review.
Cited by
- Dual roles of voltage-gated sodium channels in development and cancer.
Patel F, Brackenbury WJ. Patel F, et al. Int J Dev Biol. 2015;59(7-9):357-66. doi: 10.1387/ijdb.150171wb. Int J Dev Biol. 2015. PMID: 26009234 Free PMC article. Review. - Grey matter damage in multiple sclerosis: a pathology perspective.
Klaver R, De Vries HE, Schenk GJ, Geurts JJ. Klaver R, et al. Prion. 2013 Jan-Feb;7(1):66-75. doi: 10.4161/pri.23499. Epub 2013 Jan 1. Prion. 2013. PMID: 23324595 Free PMC article. Review. - Sodium MRI of multiple sclerosis.
Petracca M, Fleysher L, Oesingmann N, Inglese M. Petracca M, et al. NMR Biomed. 2016 Feb;29(2):153-61. doi: 10.1002/nbm.3289. Epub 2015 Apr 6. NMR Biomed. 2016. PMID: 25851455 Free PMC article. Review. - What activates inactivation?
Ahern CA. Ahern CA. J Gen Physiol. 2013 Aug;142(2):97-100. doi: 10.1085/jgp.201311046. Epub 2013 Jul 15. J Gen Physiol. 2013. PMID: 23858004 Free PMC article. No abstract available. - Secondary Progressive Multiple Sclerosis: Definition and Measurement.
Plantone D, De Angelis F, Doshi A, Chataway J. Plantone D, et al. CNS Drugs. 2016 Jun;30(6):517-26. doi: 10.1007/s40263-016-0340-9. CNS Drugs. 2016. PMID: 27166830 Review.
References
- Goldin, A. L., Barchi, R. L., Caldwell, J. H., Hofmann, F., Howe, J. R., Hunter, J. C., Kallen, R. G., Mandel, G., Meisler, M. H., Netter, Y. B., et al. (2000) Neuron 28, 365–368. - PubMed
- Boiko, T., Rasband, M. N., Levinson, S. R., Caldwell, J. H., Mandel, G., Trimmer, J. S. & Matthews, G. (2001) Neuron 30, 91–104. - PubMed
- Craner, M. J., Lo, A. C., Black, J. A. & Waxman, S. G. (2003) Brain 126, 1552–1561. - PubMed
- Waxman, S. G. (1998) N. Engl. J. Med. 338, 323–325. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous