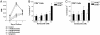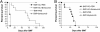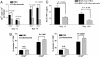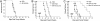Inhibition of acute graft-versus-host disease with retention of graft-versus-tumor effects by the proteasome inhibitor bortezomib - PubMed (original) (raw)
. 2004 May 25;101(21):8120-5.
doi: 10.1073/pnas.0401563101. Epub 2004 May 17.
Lisbeth A Welniak, Angela Panoskaltsis-Mortari, Matthew J O'Shaughnessy, Haiyan Liu, Isabel Barao, William Riordan, Raquel Sitcheran, Christian Wysocki, Jonathan S Serody, Bruce R Blazar, Thomas J Sayers, William J Murphy
Affiliations
- PMID: 15148407
- PMCID: PMC419567
- DOI: 10.1073/pnas.0401563101
Inhibition of acute graft-versus-host disease with retention of graft-versus-tumor effects by the proteasome inhibitor bortezomib
Kai Sun et al. Proc Natl Acad Sci U S A. 2004.
Erratum in
- Proc Natl Acad Sci U S A. 2004 Aug 24;101(34):12777
Abstract
Graft-versus-host disease (GVHD) represents a major hurdle impeding the efficacy of allogeneic bone marrow transplantation (BMT). Bortezomib is a proteasome inhibitor that was recently approved for treatment of myeloma. We found that bortezomib potently inhibited in vitro mixed lymphocyte responses and promoted the apoptosis of alloreactive T cells. Bortezomib given at the time of allogeneic BMT in mice resulted in significant protection from acute GVHD. Reductions in GVHD-associated parameters and biological evidence of proteasome inhibition were observed with this regimen but with no adverse effects on long-term donor reconstitution. Assessment of graft-versus-tumor responses in advanced leukemia-bearing mice demonstrated that only the combination of allogeneic BMT and T cells with bortezomib promoted significant increases in survival. Increased cytotoxic T cell killing of the tumor was also observed. Thus, the combination of proteasome inhibition with selective immune attack can markedly increase the efficacy of BMT in cancer.
Figures
Fig. 1.
Proliferating, and not resting, T cells are highly sensitive to bortezomib-mediated cytotoxicity. Allogeneic T cell responses are inhibited by bortezomib in vitro. (A_–_C) Proliferation and induction of apoptosis of alloreactive T cells in a MLR. (A) Alloreactive T cell proliferation was significantly decreased at days 3 and 5 (P < 0.001) in the presence of 2 nM (▾) and 4 nM (♦) but not 1 nM (▴) bortezomib. (B) Proportionally greater increase in annexin V binding on proliferating (CFSElo) compared with nonalloreactive (CFSEhi) CD4+ T cells with exposure to 4 nM bortezomib. (C) Proportionally greater increase in cell surface expression of annexin V on proliferating (CFSElo) compared with nonalloreactive (CFSEhi) CD8+ T cells with exposure to 2 and 4 nM bortezomib. *, significant differences of annexin V binding on bortezomib-exposed T cells compared with vehicle control (P < 0.05). The combined results of two independent experiments presented in B and C.
Fig. 2.
Protection of mice from acute lethal GVHD with bortezomib administration. (A) Bortezomib administration delays GVHD mortality in an aggressive model of acute lethal GVHD. B6 (H-2b) recipients of 15 million BALB/c (H-2d) bone marrow and 40 million spleen cells were treated with 10 μg per dose of bortezomib or vehicle control (PBS) daily from day 0 through day +3 post-BMT. Significant increases in survival were observed in bortezomib-treated animals (▾) compared with PBS-treated mice (▪)(P < 0.0001). (B) Bortezomib administration protects mice from GVHD mortality in a moderately aggressive model of acute lethal GVHD. B6 (H-2b) recipients of 15 million BALB/c (H-2d) bone marrow and 25 million spleen cells were treated with 10 μg per dose of bortezomib or vehicle control (PBS) daily from day 0 through +2 post-BMT. Significant increases in survival were observed in bortezomib-treated animals (▾) compared with PBS-treated mice (▾) (P < 0.0001). Results from one of three independent experiments are presented for A and B. Each experiment consists of 8–10 mice per treatment group in GVHD induction arms and 3–4 mice in BMT control arms.
Fig. 3.
Bortezomib administration reduces donor-derived T cell expansion after BMT. B6 (H-2b) recipients of 15 million BALB/c (H-2d) T cell-depleted bone marrow and 15 million CFSE-labeled purified T cells were treated with bortezomib or vehicle control (PBS) daily on day 0 and day +2 post-BMT. Each treatment group consists of three mice per group. Representative data from one of two independent experiments are presented. (A) Significant reductions (Student's t test; P < 0.05) in donor-derived CD4+ and CD8+ splenocytes were observed on days +3 and +4 post-BMT of bortezomib-treated mice. (B) Significant increases in annexin V binding on proliferating (CFSElo) but not on nonalloreactive (CFSEhi) CD4+ and CD8+ T cells in spleens from bortezomib-treated mice on day +3 post-BMT. (C) B6 (H-2b) recipients of 15 million BALB/c (H-2d) bone marrow and 20 million spleen cells were treated with bortezomib or vehicle control (PBS) daily from day 0 through day +2 post-BMT. Mice were bled on days +7 and +30 after BMT. Serum was analyzed for TNF-α as described in Materials and Methods. Significant reductions (Student's t test; P < 0.05) in serum TNF-α were observed at both time points in bortezomib-treated mice. Representative data from one of two independent experiments are presented.
Fig. 4.
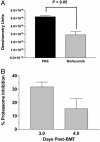
Proteasome inhibition and nuclear NF-κB activity after treatment with bortezomib in vivo. B6 mice were transplanted with BALB/c bone marrow and 25 million splenocytes as described in Materials and Methods and treated on days 0 and +2 with 20 μg of bortezomib or PBS. Spleens from two to three mice per treatment group were collected on days +3 and +4 for measurement of 20S proteasome inhibition and day +4 for nuclear NF-κB activity analysis. (A) Significant reductions in nuclear NF-κB activity in bortezomib-treated mice were measured by electromobility shift assay. (B) Inhibition of chymotrypic activity in splenic cell homogenates of bortezomib-treated mice was compared with samples from PBS-treated mice.
Fig. 5.
GVT activity is preserved with bortezomib administration after BMT. (A_–_C) B6 (H-2b) mice received 2 × 105 syngeneic C1498 cells on day 0. Effects of bortezomib administration on tumor survival were determined in various models. Mice received 15 μg per dose bortezomib or vehicle control (PBS) daily on days +11 and +13 after tumor injection. Results from one of three independent experiments are presented. Each experiment consists of 8–11 mice per treatment group. (A) No difference in survival was observed in nontransplanted mice. (B) Some groups were irradiated on day +10 after tumor cell injection, followed by injection of 15 million BALB/c (H-2d) bone marrow cells on day +11. Allogeneic BMT provided significant protection in tumor survival (P < 0.005) that was not changed by bortezomib administration. (C) In the same representative experiment as B, some groups additionally received 35 million BALB/c spleen cells. Mice that received vehicle control (PBS) injections succumbed to GVHD-associated morbidity. Mice that received bortezomib (▴) survived significantly longer than either untreated tumor-bearing mice (•; P < 0.0001) or mice that received a BMT with spleen cell and vehicle control treatment (▪; P < 0.0001).
Fig. 6.
Bortezomib enhances CTL killing of allogeneic tumor cells. BALB/c (H2d) mice were primed with irradiated C1498 cells (H2b) as described in Materials and Methods. Spleen cells were then harvested and restimulated with irradiated C1498 cells in vitro at a ratio of 20:1 for 5 days. CTL were collected and cultured with C1498 (H2b) in the presence or absence of 4 nM bortezomib. Tumor cells were analyzed by flow cytometry and clonogeneic growth. (A and B) C1498 cells were gated on based on H2b expression and forward- and side-scatter properties; the gated cells were analyzed for annexin V binding and propidium iodide incorporation at the end of 36-h incubation. (A) Representative dot plots. (B) Decreased numbers of live C1498 tumor cells (annexin V and propidium iodide negative). (C) Clonogeneic C1498 growth was determined after treatment with CTL in the presence or absence of bortezomib for 45 h. Representative data from one of two independent experiments are presented.
Similar articles
- Differential effects of proteasome inhibition by bortezomib on murine acute graft-versus-host disease (GVHD): delayed administration of bortezomib results in increased GVHD-dependent gastrointestinal toxicity.
Sun K, Wilkins DE, Anver MR, Sayers TJ, Panoskaltsis-Mortari A, Blazar BR, Welniak LA, Murphy WJ. Sun K, et al. Blood. 2005 Nov 1;106(9):3293-9. doi: 10.1182/blood-2004-11-4526. Epub 2005 Jun 16. Blood. 2005. PMID: 15961519 Free PMC article. - Phase II clinical trial for the evaluation of bortezomib within the reduced intensity conditioning regimen (RIC) and post-allogeneic transplantation for high-risk myeloma patients.
Caballero-Velázquez T, López-Corral L, Encinas C, Castilla-Llorente C, Martino R, Rosiñol L, Sampol A, Caballero D, Serrano D, Heras I, San Miguel J, Pérez-Simón JA. Caballero-Velázquez T, et al. Br J Haematol. 2013 Aug;162(4):474-82. doi: 10.1111/bjh.12410. Epub 2013 Jun 15. Br J Haematol. 2013. PMID: 23772672 Clinical Trial. - The protection and therapy effects of bortezomib in murine acute graft-versus-host disease.
Li Z, Wu Q, Yan Z, Li D, Lu G, Mou W, Wu S, Pan X, Lu Q, Xu K. Li Z, et al. Transplant Proc. 2013 Jul-Aug;45(6):2527-35. doi: 10.1016/j.transproceed.2013.03.042. Transplant Proc. 2013. PMID: 23953575 - [Bortezomib in multiple myeloma patients after allogeneic stem cell transplantation].
Vokurka S. Vokurka S. Klin Onkol. 2010;23(4):242-4. Klin Onkol. 2010. PMID: 20806822 Review. Czech. - Proteasome inhibition in transplantation-focusing on the experience with bortezomib.
Liang Y, Liu H. Liang Y, et al. Curr Pharm Des. 2013;19(18):3299-304. doi: 10.2174/13816128113199990308. Curr Pharm Des. 2013. PMID: 23151133 Review.
Cited by
- Cutting edge of immune response and immunosuppressants in allogeneic and xenogeneic islet transplantation.
Yue L, Li J, Yao M, Song S, Zhang X, Wang Y. Yue L, et al. Front Immunol. 2024 Sep 13;15:1455691. doi: 10.3389/fimmu.2024.1455691. eCollection 2024. Front Immunol. 2024. PMID: 39346923 Free PMC article. Review. - Tetramerization of pyruvate kinase M2 attenuates graft-versus-host disease by inhibition of Th1 and Th17 differentiation.
Wang M, Li QJ, Zhao HY, Zhang JL. Wang M, et al. Hum Cell. 2024 May;37(3):633-647. doi: 10.1007/s13577-024-01033-6. Epub 2024 Feb 28. Hum Cell. 2024. PMID: 38416276 - Graft-versus-host disease: teaching old drugs new tricks at less cost.
Farhan S, Holtan SG. Farhan S, et al. Front Immunol. 2023 Aug 3;14:1225748. doi: 10.3389/fimmu.2023.1225748. eCollection 2023. Front Immunol. 2023. PMID: 37600820 Free PMC article. Review. - Maintenance therapy after allogeneic hematopoietic stem cell transplantation for patients with multiple myeloma.
Kawamura K. Kawamura K. Int J Hematol. 2023 Aug;118(2):193-200. doi: 10.1007/s12185-023-03602-1. Epub 2023 Apr 15. Int J Hematol. 2023. PMID: 37060508 Review.
References
- Ferrara, J. L., Deeg, H. & Burakoff, S. J. (1997) Graft-versus-Host Disease (Marcel Decker, New York).
- Graubert, T. A., Russell, J. H. & Ley, T. J. (1996) Blood 87, 1232–1237. - PubMed
- Nestel, F. P., Greene, R. N., Kichian, K., Ponka, P. & Lapp, W. S. (2000) Blood 96, 1836–1843. - PubMed
- Welniak, L. A., Blazar, B. R., Wiltrout, R. H., Anver, M. R. & Murphy, W. J. (2001) Transplant. Proc. 33, 1752–1753. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2 R37 HL56067/HL/NHLBI NIH HHS/United States
- R01 CA102282/CA/NCI NIH HHS/United States
- R01 AI 34495/AI/NIAID NIH HHS/United States
- R01 AI034495/AI/NIAID NIH HHS/United States
- R01 CA72669/CA/NCI NIH HHS/United States
- R37 HL056067/HL/NHLBI NIH HHS/United States
- N01CO12400/CA/NCI NIH HHS/United States
- R01 CA072669/CA/NCI NIH HHS/United States
- N01-CO-12400/CO/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources