The evolution of a pleiotropic fitness tradeoff in Pseudomonas fluorescens - PubMed (original) (raw)
The evolution of a pleiotropic fitness tradeoff in Pseudomonas fluorescens
R Craig MacLean et al. Proc Natl Acad Sci U S A. 2004.
Abstract
The evolution of ecological specialization is expected to carry a cost, due to either antagonistic pleiotropy or mutation accumulation. In general, it has been difficult to distinguish between these two possibilities. Here, we demonstrate that the experimental evolution of niche-specialist genotypes of the bacterium Pseudomonas fluorescens that colonize the air-broth interface of spatially structured microcosms is accompanied by pleiotropic fitness costs in terms of reduced carbon catabolism. Prolonged selection in spatially structured microcosms caused the cost of specialization to decline without loss of the benefits associated with specialization. The decline in the cost of specialization can be explained by either compensatory adaptation within specialist lineages or clonal competition among specialist lineages. These results provide a possible explanation of conflicting accounts for the cost of specialization.
Figures
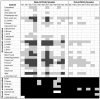
Fig. 1.
Biolog profile of WS genotypes. The substrates shown here either always support the growth of the ancestral SM genotype (ancestral substrates) or never support the growth of this genotype (novel substrates). Blank boxes, substrates that support substantial growth of WS genotypes; gray boxes, substrates that support weak growth of WS genotypes; black boxes, substrates that do not support visible growth of WS genotypes.
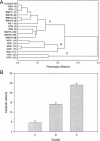
Fig. 2.
The evolution of the WS metabolic profile. (A) Phenotypic clustering of WS genotypes and ancestral SM. Each tip corresponds to a single genotype. WS genotypes from continued selection lines are in bold. Numbers in brackets denote the number of catabolic defects expressed by each genotype, such that the value of ancestral SM is 0 by definition. Letters (A, B, and C) denote the three principle phenotypic clusters. (B) Mean (± SE) number catabolic defects of WS genotypes belonging to the three principle clusters identified in A.
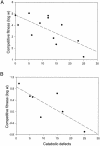
Fig. 3.
Competitive fitness and catabolic performance. Plotted points show the competitive fitness of WS genotypes that evolve after 1 week of selection and catabolic defects, measured as the number of ancestral Biolog substrates that fail to support vigorous growth of WS genotypes. Dashed lines show linear regressions of fitness on catabolic performance. (A) Fitness was assayed as the ability of a WS genotype to invade a population of the ancestral broth-colonizing genotype from an initial frequency of ≈1% over a 2-day competition period. (B) Fitness was assayed as the competitive ability of a WS genotype against a randomly chosen WS tester genotype at an initial frequency of 1:1 over a 1-day competition period.
Similar articles
- Adaptive divergence in experimental populations of Pseudomonas fluorescens. V. Insight into the niche specialist fuzzy spreader compels revision of the model Pseudomonas radiation.
Ferguson GC, Bertels F, Rainey PB. Ferguson GC, et al. Genetics. 2013 Dec;195(4):1319-35. doi: 10.1534/genetics.113.154948. Epub 2013 Sep 27. Genetics. 2013. PMID: 24077305 Free PMC article. - Adaptation limits diversification of experimental bacterial populations.
Buckling A, Wills MA, Colegrave N. Buckling A, et al. Science. 2003 Dec 19;302(5653):2107-9. doi: 10.1126/science.1088848. Science. 2003. PMID: 14684817 - Adaptive radiation of Pseudomonas fluorescens SBW25 in experimental microcosms provides an understanding of the evolutionary ecology and molecular biology of A-L interface biofilm formation.
Koza A, Kusmierska A, McLaughlin K, Moshynets O, Spiers AJ. Koza A, et al. FEMS Microbiol Lett. 2017 Jul 3;364(12). doi: 10.1093/femsle/fnx109. FEMS Microbiol Lett. 2017. PMID: 28535292 Review. - On the experimental evolution of specialization and diversity in heterogeneous environments.
Jasmin JN, Kassen R. Jasmin JN, et al. Ecol Lett. 2007 Apr;10(4):272-81. doi: 10.1111/j.1461-0248.2007.01021.x. Ecol Lett. 2007. PMID: 17355566 - Evolvability Costs of Niche Expansion.
Bono LM, Draghi JA, Turner PE. Bono LM, et al. Trends Genet. 2020 Jan;36(1):14-23. doi: 10.1016/j.tig.2019.10.003. Epub 2019 Nov 5. Trends Genet. 2020. PMID: 31699305 Review.
Cited by
- Phage-resistant mutations impact bacteria susceptibility to future phage infections and antibiotic response.
McGee LW, Barhoush Y, Shima R, Hennessy M. McGee LW, et al. Ecol Evol. 2023 Jan 6;13(1):e9712. doi: 10.1002/ece3.9712. eCollection 2023 Jan. Ecol Evol. 2023. PMID: 36620417 Free PMC article. - In situ phylogenetic structure and diversity of wild Bradyrhizobium communities.
Sachs JL, Kembel SW, Lau AH, Simms EL. Sachs JL, et al. Appl Environ Microbiol. 2009 Jul;75(14):4727-35. doi: 10.1128/AEM.00667-09. Epub 2009 May 29. Appl Environ Microbiol. 2009. PMID: 19482951 Free PMC article. - The cost of evolved constitutive lac gene expression is usually, but not always, maintained during evolution of generalist populations.
Phillips KN, Cooper TF. Phillips KN, et al. Ecol Evol. 2021 Aug 6;11(18):12497-12507. doi: 10.1002/ece3.7994. eCollection 2021 Sep. Ecol Evol. 2021. PMID: 34594515 Free PMC article. - Interaction between mutation type and gene pleiotropy drives parallel evolution in the laboratory.
Ruelens P, Wynands T, de Visser JAGM. Ruelens P, et al. Philos Trans R Soc Lond B Biol Sci. 2023 May 22;378(1877):20220051. doi: 10.1098/rstb.2022.0051. Epub 2023 Apr 3. Philos Trans R Soc Lond B Biol Sci. 2023. PMID: 37004729 Free PMC article. - Effective specialist or jack of all trades? Experimental evolution of a crop pest in fluctuating and stable environments.
Skoracka A, Laska A, Radwan J, Konczal M, Lewandowski M, Puchalska E, Karpicka-Ignatowska K, Przychodzka A, Raubic J, Kuczyński L. Skoracka A, et al. Evol Appl. 2022 Mar 8;15(10):1639-1652. doi: 10.1111/eva.13360. eCollection 2022 Oct. Evol Appl. 2022. PMID: 36330306 Free PMC article.
References
- Levene, H. (1953) Am. Nat. 88, 690–692.
- Levins, R. (1968) Evolution in Changing Environments (Princeton Univ. Press, Princeton).
- Chesson, P. (2000) Annu. Rev. Ecol. Syst. 31, 343–366.
- Lynch, M. & Gabriel, W. (1987) Am. Nat. 129, 282–303.
- Roughgarden, J. (1972) Am. Nat. 106, 683–718.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources