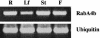The Arabidopsis Rab GTPase RabA4b localizes to the tips of growing root hair cells - PubMed (original) (raw)
. 2004 Jun;16(6):1589-603.
doi: 10.1105/tpc.021634. Epub 2004 May 21.
Affiliations
- PMID: 15155878
- PMCID: PMC490048
- DOI: 10.1105/tpc.021634
The Arabidopsis Rab GTPase RabA4b localizes to the tips of growing root hair cells
Mary L Preuss et al. Plant Cell. 2004 Jun.
Abstract
Spatial and temporal control of cell wall deposition plays a unique and critical role during growth and development in plants. To characterize membrane trafficking pathways involved in these processes, we have examined the function of a plant Rab GTPase, RabA4b, during polarized expansion in developing root hair cells. Whereas a small fraction of RabA4b cofractionated with Golgi membrane marker proteins, the majority of this protein labeled a unique membrane compartment that did not cofractionate with the previously characterized trans-Golgi network syntaxin proteins SYP41 and SYP51. An enhanced yellow fluorescent protein (EYFP)-RabA4b fusion protein specifically localizes to the tips of growing root hair cells in Arabidopsis thaliana. Tip-localized EYFP-RabA4b disappears in mature root hair cells that have stopped expanding, and polar localization of the EYFP-RabA4b is disrupted by latrunculin B treatment. Loss of tip localization of EYFP-RabA4b was correlated with inhibition of expansion; upon washout of the inhibitor, root hair expansion recovered only after tip localization of the EYFP-RabA4b compartments was reestablished. Furthermore, in mutants with defective root hair morphology, EYFP-RabA4b was improperly localized or was absent from the tips of root hair cells. We propose that RabA4b regulates membrane trafficking through a compartment involved in the polarized secretion of cell wall components in plant cells.
Copyright 2004 American Society of Plant Biologists
Figures
Figure 1.
RabA4b Is Expressed Ubiquitously in A. thaliana Plants. Tissue from roots (R), leaves (Lf), stems (St), and flowers (F) of 3-week-old A. thaliana plants were used for RNA isolation. RT-PCR analysis of RabA4b expression was performed with primers specific for RabA4b and primers specific to ubiquitin as a loading control.
Figure 2.
Promoter:EYFP Analysis of RabA4b Expression in A. thaliana. Seven- to ten-day-old seedlings expressing the RabA4b promoter:EYFP gene were imaged using a Zeiss M2-Bio fluorescence dissecting scope with a 1.0× lens either with epifluorescence illumination and appropriate EYFP filters ([A], [B], and [C]) or with transmitted light ([D], [E], and [F]). EYFP fluorescence was observed throughout the shoots ([A] and [D]) and roots ([B] and [E]) and also in root hair cells ([C] and [F]).
Figure 3.
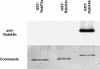
Specificity of the RabA4b Antibody. Antisera raised to RabA4b was purified and tested on a protein blot with equal quantities (0.1 μg of protein) of recombinant RabA4b, RabAF2a, and RabG3c protein. The antibodies recognized only RabA4b and not other Rab proteins under these conditions.
Figure 4.
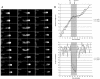
RabA4b Is Localized to a Novel Membrane Compartment. (A) Transformed A. thaliana plants were generated expressing EYFP-RabA4b, GFP-PD3-5c (Cutler, 2001), or GFP-180598E (Cutler et al., 2000) under control of the 35S promoter of Cauliflower mosaic virus. Membranes were isolated from roots of 2- to 3-week-old untransformed seedlings grown in liquid culture under continuous shaking and then separated by sucrose density gradient fractionation (20 to 60%, w/v). Fractions (500 μL) were collected from the top (fraction 2) to the bottom (fraction 24), and proteins were analyzed by SDS-PAGE followed by immunoblotting with specific antibodies. Anti-RabA4b antibodies were used to detect membranes containing endogenously expressed RabA4b. In transformed plants, membranes containing EYFP-RabA4b and endogenous RabA4b were detected by immunoblotting with antibodies specific for EYFP and RabA4b, respectively. Cofractionation of these two proteins is consistent with localization of the EYFP-RabA4b fusion protein to membranes that also contain endogenously expressed RabA4b. RabA4b from wild-type plants also cofractionated with EYFP-RabA4b, showing that expression of the fusion protein did not alter the nature of this compartment. To detect Golgi membranes, antibodies specific to Golgi-localized α-1,2-mannosidase I were used. Interestingly, this antibody recognized two distinct proteins: a lower molecular mass protein (63.5 kD) that cofractionated with two other Golgi marker proteins, PD3-5c and 180598E (Cutler et al., 2000; Cutler, 2001), and a higher molecular mass protein (66 kD) that cofractionated with two TGN markers, SYP41 (Bassham et al., 2000) and SYP51 (Sanderfoot et al., 2001). EYFP-RabA4b–containing membranes displayed a fractionation pattern similar to, but distinct from, membranes containing these TGN markers, SYP41 and SYP51. RabA4b and EYFP-RabA4b also did not cofractionate with AtSec12, an ER-localized protein (Bar-Peled and Raikhel, 1997), or SYP21, a syntaxin localized to endosomes (Sanderfoot et al., 1998). (B) To quantitatively measure the fractionation patterns of the various proteins in this analysis, band intensities were first collected for each immunoblot. These measurements were then normalized to compensate for overall variation in band intensities observed with the different antibodies. After normalization, these values were plotted for quantitative comparison of fractionation profiles.
Figure 5.
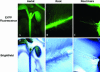
EYFP-RabA4b Localizes to the Tips of Root Hairs in A. thaliana. Root hairs of A. thaliana seedlings expressing EYFP-RabA4b ([A], [C], [E], and [G]) or EYFP-RabF2a ([B], [D], [F], and [H]) were imaged at high magnification using a Zeiss M2-Bio fluorescence dissecting scope with a 1.0× lens either with transmitted light ([A] and [B]) or with epifluorescence illumination and appropriate EYFP filters ([C] and [D]). Arrows indicate the EYFP-RabA4b localized to the extreme tips of root hair cells ([A] and [C]). By contrast, EYFP-RabF2a was not preferentially localized to root hair tips ([B] and [D], arrowheads); rather, it was distributed in punctate structures along the length of the root hair ([D], arrows). Lower magnification images show the zone of growing root hairs where EYFP-RabA4b is tip localized ([E] and [G]) and EYFP-RabF2a is distributed along the length of each root hair ([F] and [H]).
Figure 6.
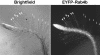
Only Growing Root Hairs Display Polarized EYFP-RabA4b. Images of A. thaliana seedlings stably expressing EYFP-RabA4b were collected using a Zeiss M2-Bio fluorescence dissecting microscope equipped with a 1.0× lens. Successive images were collected either with transmitted light, (brightfield, left panel) or with epifluorescence illumination and appropriate EYFP filters (EYFP-RabA4b, right panel). Root hairs in the vicinity of the root meristem display tip-localized EYFP-RabA4b compartments (arrowheads), whereas the tip localization of EYFP-RabA4b was not seen in mature root hair cells (arrows).
Figure 7.
Treatment with the Actin-Depolymerizing Drug LB Causes Loss of EYFP-RabA4b Tip Localization in Root Hairs. (A) Seedlings expressing EYFP-RabA4b were grown in liquid media and transferred to a perfusion chamber for fluorescence microscopy. Normal root hair expansion was observed for 20 min. A dotted line denotes the relative position of the root hair tip at the beginning of analysis. Upon treatment with 200 nM LB (20 min time point), EYFP-RabA4b tip localization was rapidly lost, and fluorescence was observed along the entire length of the cell (24 to 30 min time points). This effect was reversible: washout of LB (28 min time point) resulted in reorganization of tip-localized EYFP-RabA4b after a short lag (34 min time point). (B) Quantitative analysis of LB inhibition of root hair growth. Root hair length and EYFP-RabA4b tip fluorescence were quantified in two representative root hairs (RH1 and RH2) treated with LB. Root hair fluorescence was quantified using computational methods. Fluorescent signal located within the proximal 15% of the length of the root hair was defined as tip fluorescence, and this was presented as a percentage of the fluorescence detected in the entire root hair. Growth of each root hair was inhibited when tip fluorescence was lost. Upon recovery of tip fluorescence, root hair expansion resumed. Shaded area denotes the time period in which tip fluorescence was absent.
Figure 8.
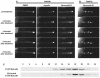
Tip Localization of the EYFP-RabA4b Compartment Does Not Require Intact Microtubules. (A) Root hairs treated with LB show significant loss of tip localization within 2 min of treatment. In the same time frame, root hairs treated with 10 μM oryzalin to depolymerize microtubules and control treatment with DMSO display no obvious defects in tip localization. (B) In contrast with root hairs treated with EYFP-RabA4b, the overall distribution of EYFP-RabF2a in root hairs was not significantly changed upon treatment with LB. (C) LB treatment did not significantly alter the characteristics of EYFP-RabA4b–labeled compartments. Plants expressing EYFP-RabA4b were treated with LB for 15 min before subcellular fractionation of the membranes over a sucrose density gradient. The fractionation pattern of EYFP-RabA4b in membranes treated with LB was similar to that in nontreated plants (cf. with fractionation patterns in Figure 4). These results demonstrate that tip localization of RabA4b compartments is dependent on an intact actin cytoskeleton and that the nature of the compartment is not demonstrably changed by LB treatment.
Figure 9.
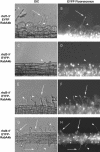
Localization of the EYFP-RabA4b Fluorescence in the rhd Mutant Backgrounds. The 35S-EYFP-RabA4b construct was transformed into the mutant root hair lines rhd1-1 ([A] and [B]), rhd2-1 ([C] and [D]), rhd3-1 ([E] and [F]), and rhd4-1 ([G] and [H]). Plants were grown in 0.25× MS + 0.3% phytagel and transferred to microscope slides. Root hairs were observed using a Nikon Eclipse E600 microscope with differential interference contrast ([A], [C], [E], and [G]) and epifluorescence ([B], [D], [F], and [H]) optics. Arrows indicate root hairs with normal tip localization of EYFP-RabA4b. Arrowheads point to abnormal distributions of the EYFP-RabA4b in root hairs.
Similar articles
- A role for the RabA4b effector protein PI-4Kbeta1 in polarized expansion of root hair cells in Arabidopsis thaliana.
Preuss ML, Schmitz AJ, Thole JM, Bonner HK, Otegui MS, Nielsen E. Preuss ML, et al. J Cell Biol. 2006 Mar 27;172(7):991-8. doi: 10.1083/jcb.200508116. J Cell Biol. 2006. PMID: 16567499 Free PMC article. - Trans-Golgi network localized small GTPase RabA1d is involved in cell plate formation and oscillatory root hair growth.
Berson T, von Wangenheim D, Takáč T, Šamajová O, Rosero A, Ovečka M, Komis G, Stelzer EH, Šamaj J. Berson T, et al. BMC Plant Biol. 2014 Sep 27;14:252. doi: 10.1186/s12870-014-0252-0. BMC Plant Biol. 2014. PMID: 25260869 Free PMC article. - Root hair defective4 encodes a phosphatidylinositol-4-phosphate phosphatase required for proper root hair development in Arabidopsis thaliana.
Thole JM, Vermeer JE, Zhang Y, Gadella TW Jr, Nielsen E. Thole JM, et al. Plant Cell. 2008 Feb;20(2):381-95. doi: 10.1105/tpc.107.054304. Epub 2008 Feb 15. Plant Cell. 2008. PMID: 18281508 Free PMC article. - Vesicular trafficking, cytoskeleton and signalling in root hairs and pollen tubes.
Samaj J, Müller J, Beck M, Böhm N, Menzel D. Samaj J, et al. Trends Plant Sci. 2006 Dec;11(12):594-600. doi: 10.1016/j.tplants.2006.10.002. Epub 2006 Nov 7. Trends Plant Sci. 2006. PMID: 17092761 Review. - Building a hair: tip growth in Arabidopsis thaliana root hairs.
Carol RJ, Dolan L. Carol RJ, et al. Philos Trans R Soc Lond B Biol Sci. 2002 Jun 29;357(1422):815-21. doi: 10.1098/rstb.2002.1092. Philos Trans R Soc Lond B Biol Sci. 2002. PMID: 12079677 Free PMC article. Review.
Cited by
- The proteolytic processing of seed storage proteins in Arabidopsis embryo cells starts in the multivesicular bodies.
Otegui MS, Herder R, Schulze J, Jung R, Staehelin LA. Otegui MS, et al. Plant Cell. 2006 Oct;18(10):2567-81. doi: 10.1105/tpc.106.040931. Epub 2006 Sep 29. Plant Cell. 2006. PMID: 17012602 Free PMC article. - ROS Signaling Pathways in Chilling Stress.
Einset J, Winge P, Bones A. Einset J, et al. Plant Signal Behav. 2007 Sep;2(5):365-7. doi: 10.4161/psb.2.5.4461. Plant Signal Behav. 2007. PMID: 19704600 Free PMC article. - FRET imaging in living maize cells reveals that plasma membrane aquaporins interact to regulate their subcellular localization.
Zelazny E, Borst JW, Muylaert M, Batoko H, Hemminga MA, Chaumont F. Zelazny E, et al. Proc Natl Acad Sci U S A. 2007 Jul 24;104(30):12359-64. doi: 10.1073/pnas.0701180104. Epub 2007 Jul 16. Proc Natl Acad Sci U S A. 2007. PMID: 17636130 Free PMC article. - The Arabidopsis AAA ATPase SKD1 is involved in multivesicular endosome function and interacts with its positive regulator LYST-INTERACTING PROTEIN5.
Haas TJ, Sliwinski MK, Martínez DE, Preuss M, Ebine K, Ueda T, Nielsen E, Odorizzi G, Otegui MS. Haas TJ, et al. Plant Cell. 2007 Apr;19(4):1295-312. doi: 10.1105/tpc.106.049346. Epub 2007 Apr 27. Plant Cell. 2007. PMID: 17468262 Free PMC article. - Ectopic expression of an activated RAC in Arabidopsis disrupts membrane cycling.
Bloch D, Lavy M, Efrat Y, Efroni I, Bracha-Drori K, Abu-Abied M, Sadot E, Yalovsky S. Bloch D, et al. Mol Biol Cell. 2005 Apr;16(4):1913-27. doi: 10.1091/mbc.e04-07-0562. Epub 2005 Feb 9. Mol Biol Cell. 2005. PMID: 15703216 Free PMC article.
References
- Baluska, F., Salaj, J., Mathur, J., Braun, M., Jasper, F., Samaj, J., Chua, N.H., Barlow, P.W., and Volkmann, D. (2000). Root hair formation: F-actin-dependent tip growth is initiated by local assembly of profilin-supported F-actin meshworks accumulated within expansin-enriched bulges. Dev. Biol. 227, 618–632. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases