Lethality to human cancer cells through massive chromosome loss by inhibition of the mitotic checkpoint - PubMed (original) (raw)
Lethality to human cancer cells through massive chromosome loss by inhibition of the mitotic checkpoint
Geert J P L Kops et al. Proc Natl Acad Sci U S A. 2004.
Abstract
A compromised mitotic checkpoint, the primary mechanism for ensuring that each new cell receives one copy of every chromosome, has been implicated as a contributor to carcinogenesis. However, a checkpoint response is shown here to be essential for cell survival, including that of chromosomally instable colorectal cancer cells. Reducing the levels of the checkpoint proteins BubR1 or Mad2 in human cancer cells or inhibiting BubR1 kinase activity provokes apoptotic cell death within six divisions except when cytokinesis is also inhibited. Thus, suppression of mitotic checkpoint signaling is invariably lethal as the consequence of massive chromosome loss, findings that have implications for inhibiting proliferation of tumor cells.
Figures
Fig. 1.
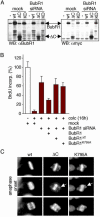
Absence of mitotic checkpoint response in BubR1- or Mad2-depleted cells. (A) HeLa cells transfected with the indicated siRNA plasmids were treated with or without colcemid for 16 hr, and whole-cell lysates of the transfected population were immunoblotted for cyclin B1, BubR1, or actin. _p_-BubR1, phosphorylated BubR1. (B) Stills of time-lapse movies 1–3. Asterisks indicate nuclear envelope breakdown. (C) Time-lapse sequence of cells transfected with Mad2 siRNA plasmid and pH2B-EYFP. Arrows indicate the reassembled nuclear envelope. (D) T98G cells transfected with the indicated siRNA plasmids were treated with or without colcemid for 16 hr, and the entire population was analyzed for BrdUrd incorporation. S phase indicates the percentage of the cell population that is BrdUrd positive. (E) DNA content profiles of T98G cells that were transfected and treated as in D.(F) Stills of time-lapse movies 4–6. (G) HeLa cells were transfected with the indicated siRNA plasmids. Forty-eight hours posttransfection, transfected cells were isolated by magnetic activated cell sorting and replated onto coverslips. Twenty hours after replating, cells were fixed and DNA was visualized with 4′,6-diamidino-2-phenylindole (DAPI).
Fig. 2.
BubR1 kinase activity is required for mitotic checkpoint signaling. (A) Total cellular lysates of T98G cells transfected with mock or BubR1 siRNA plasmid in combination with either empty vector or myc-tagged siRNA-resistant BubR1 mutants were immunoblotted for BubR1 or the myc epitope tag. KD, K795A. (B) T98G cells were transfected, treated, and analyzed as in Fig. 1_D_, with the addition of the various siRNA-resistant BubR1 alleles during transfection. Percentage BrdUrd positivity in the mock samples without colcemid was set to 100. (C) Stills of live cell microscopy of BubR1 siRNA HeLa cells transfected with pH2B-EYFP and the indicated siRNA-resistant BubR1 alleles. Forty-eight hours posttransfection, images of H2B-EYFP-positive cells were taken at 2-min intervals. Arrows indicate unaligned chromosomes during anaphase.
Fig. 3.
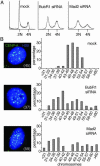
Mitosis with reduced BubR1 or Mad2 causes acute chromosome loss. (A) DNA content profiles of HeLa cells transfected with the indicated siRNA plasmids along with pBabe-Puro for 24 hr and grown in puromycin-containing medium for an additional 48 hr. Nontransfected cells were removed 14 hr before analysis. (B) Distribution of the amount of chromosomes within a G1 population of YCA-2A3 cells transfected with mock, BubR1 siRNA, or Mad2 siRNA. Images are Z-stack projections displaying all EYFP-CENP-A-containing centromeres in one plane of a transfected (H2B-ECFP-positive) cell. Number in parentheses indicates number of chromosomes.
Fig. 4.
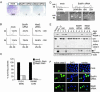
Loss of viability by inhibition of mitotic checkpoint signaling. (A) Colony outgrowth assay. Indicated is the number of colonies on each plate. (B) HeLa cells transfected with the indicated siRNAs along with pBabe-Puro were analyzed for DNA content after 4 or 5 days of growth in puromycin-containing medium. The extent of cell death is shown as percentage of cells with sub2N DNA content. (C) Colony outgrowth assay of DLD-1 and SW480 cells. Percentage of surviving colonies in mock transfected samples was set to 100. (D) HeLa cells transfected as in B were analyzed for DNA content (Upper) and morphology (Lower) after growth in puromycin- and colcemid-containing medium for an additional 3 or 6 days, respectively. (Bar = 50 μm.) (E) Whole-cell extracts of HeLa cells transfected with the indicated siRNA plasmids were immunoblotted for p85 poly-(ADP-ribose) polymerase-1 (PARP-1) protein cleavage product, caspase-3, or actin. Asynchronous and puromycin- and colcemid-treated cells were used as controls. (F) HeLa cells were transfected and selected as in B. Immunostaining of active caspase-3 was done 3 days posttransfection.
Fig. 5.
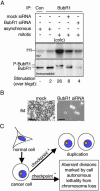
Loss of viability by partial inhibition of the essential BubR1 kinase. (A) Mitotic T98G cells, untransfected or transfected with mock or BubR1 siRNA plasmids, were analyzed for BubR1 protein levels (immunoblot) and BubR1 kinase activity (32P) by immunoprecipitation (IP). Asynchronous populations, a colcemid-treated population (10 hr; colc) and immunoprecipitation with control antibody (Con) were used as controls. (B) T98G cells were transfected as in Fig. 5_A_ and grown for 6 days. (Bar = 50 μm.) (C) Proposed effects of mitotic checkpoint status on proliferation and survival of human cancer cells. Although a weakened mitotic checkpoint may contribute to carcinogenesis, tumor cells require checkpoint signaling for proliferation. More severe rates of chromosome loss by functionally inhibiting the mitotic checkpoint, however, are lethal.
Similar articles
- Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores.
Ditchfield C, Johnson VL, Tighe A, Ellston R, Haworth C, Johnson T, Mortlock A, Keen N, Taylor SS. Ditchfield C, et al. J Cell Biol. 2003 Apr 28;161(2):267-80. doi: 10.1083/jcb.200208091. J Cell Biol. 2003. PMID: 12719470 Free PMC article. - 6,7-Dimethoxy-3-(3-methoxyphenyl)isoquinolin-1-amine induces mitotic arrest and apoptotic cell death through the activation of spindle assembly checkpoint in human cervical cancer cells.
Chung KS, Choi HE, Shin JS, Cho YW, Choi JH, Cho WJ, Lee KT. Chung KS, et al. Carcinogenesis. 2013 Aug;34(8):1852-60. doi: 10.1093/carcin/bgt133. Epub 2013 Apr 24. Carcinogenesis. 2013. PMID: 23615402 - Simultaneous reduction of MAD2 and BUBR1 expression induces mitotic spindle alterations associated with p53 dependent cell cycle arrest and death.
Lentini L, Piscitello D, Veneziano L, Di Leonardo A. Lentini L, et al. Cell Biol Int. 2014 Aug;38(8):933-41. doi: 10.1002/cbin.10277. Epub 2014 Apr 10. Cell Biol Int. 2014. PMID: 24687487 - Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2.
Martin-Lluesma S, Stucke VM, Nigg EA. Martin-Lluesma S, et al. Science. 2002 Sep 27;297(5590):2267-70. doi: 10.1126/science.1075596. Science. 2002. PMID: 12351790 - MAD2 dependent mitotic checkpoint defects in tumorigenesis and tumor cell death: a double edged sword.
Michel L, Benezra R, Diaz-Rodriguez E. Michel L, et al. Cell Cycle. 2004 Aug;3(8):990-2. Epub 2004 Aug 25. Cell Cycle. 2004. PMID: 15254432 Review.
Cited by
- Chromosomal heterogeneity and instability characterize pediatric medulloblastoma cell lines and affect neoplastic phenotype.
Castro-Gamero AM, Borges KS, Lira RC, Andrade AF, Fedatto PF, Cruzeiro GA, Silva RB, Fontes AM, Valera ET, Bobola M, Scrideli CA, Tone LG. Castro-Gamero AM, et al. Cytotechnology. 2013 Oct;65(5):871-85. doi: 10.1007/s10616-012-9529-z. Epub 2013 Jan 17. Cytotechnology. 2013. PMID: 23325114 Free PMC article. - Inducible microRNA expression by an all-in-one episomal vector system.
Epanchintsev A, Jung P, Menssen A, Hermeking H. Epanchintsev A, et al. Nucleic Acids Res. 2006;34(18):e119. doi: 10.1093/nar/gkl624. Epub 2006 Sep 22. Nucleic Acids Res. 2006. PMID: 16998186 Free PMC article. - Spindle checkpoint function and cellular sensitivity to antimitotic drugs.
Yamada HY, Gorbsky GJ. Yamada HY, et al. Mol Cancer Ther. 2006 Dec;5(12):2963-9. doi: 10.1158/1535-7163.MCT-06-0319. Mol Cancer Ther. 2006. PMID: 17172401 Free PMC article. Review. No abstract available. - Nuclear envelope, chromatin organizers, histones, and DNA: The many achilles heels exploited across cancers.
Balaji AK, Saha S, Deshpande S, Poola D, Sengupta K. Balaji AK, et al. Front Cell Dev Biol. 2022 Dec 16;10:1068347. doi: 10.3389/fcell.2022.1068347. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36589746 Free PMC article. Review. - Chromosome Missegregation as a Modulator of Radiation Sensitivity.
Cosper PF, Copeland SE, Tucker JB, Weaver BA. Cosper PF, et al. Semin Radiat Oncol. 2022 Jan;32(1):54-63. doi: 10.1016/j.semradonc.2021.09.002. Semin Radiat Oncol. 2022. PMID: 34861996 Free PMC article. Review.
References
- Lengauer, C., Kinzler, K. W. & Vogelstein, B. (1997) Nature 386, 623–627. - PubMed
- Lengauer, C., Kinzler, K. W. & Vogelstein, B. (1998) Nature 396, 643–649. - PubMed
- Cahill, D. P., Lengauer, C., Yu, J., Riggins, G. J., Willson, J. K., Markowitz, S. D., Kinzler, K. W. & Vogelstein, B. (1998) Nature 392, 300–303. - PubMed
- Shichiri, M., Yoshinaga, K., Hisatomi, H., Sugihara, K. & Hirata, Y. (2002) Cancer Res. 62, 13–17. - PubMed
- Ru, H. Y., Chen, R. L., Lu, W. C. & Chen, J. H. (2002) Oncogene 21, 4673–4679. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources