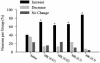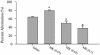NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex - PubMed (original) (raw)
NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex
Mark E Jackson et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Cognitive deficits associated with frontal lobe dysfunction are a determinant of long-term disability in schizophrenia and are not effectively treated with available medications. Clinical studies show that many aspects of these deficits are transiently induced in healthy individuals treated with N-methyl-D-aspartate (NMDA) antagonists. These findings and recent genetic linkage studies strongly implicate NMDA receptor deficiency in schizophrenia and suggest that reversing this deficiency is pertinent to treating the cognitive symptoms of schizophrenia. Despite the wealth of behavioral data on the effects of NMDA antagonist treatment in humans and laboratory animals, there is a fundamental lack of understanding about the mechanisms by which a general state of NMDA deficiency influences the function of cortical neurons. Using ensemble recording in freely moving rats, we found that NMDA antagonist treatment, at doses that impaired working memory, potentiated the firing rate of most prefrontal cortex neurons. This potentiation, which correlated with expression of behavioral stereotypy, resulted from an increased number of irregularly discharged single spikes. Concurrent with the increase in spike activity, there was a significant reduction in organized bursting activity. These results identify two distinct mechanisms by which NMDA receptor deficiency may disrupt frontal lobe function: an increase in disorganized spike activity, which may enhance cortical noise and transmission of disinformation; and a decrease in burst activity, which reduces transmission efficacy of cortical neurons. These findings provide a physiological basis for the NMDA receptor deficiency model of schizophrenia and may clarify the nature of cortical dysfunction in this disease.
Figures
Fig. 1.
Percentage of neurons with increase, decrease, or no change in firing rate. There was a significant increase in the percentage of neurons with postinjection increases in firing rate after MK801 (0.01 mg/kg, 0.05 mg/kg, 0.1 mg/kg, and 0.3 mg/kg) compared to saline. Asterisks indicate significant difference by χ2 (P < 0.01) compared to saline. Note that this pattern of response did not generalize to other psychoactive drugs such as amphetamine (Fig. 7, which is published as
supporting information
on the PNAS web site).
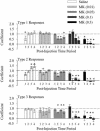
Fig. 2.
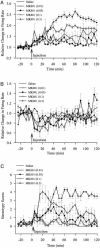
Temporal profile of firing rate changes and behavioral activity after systemic MK801 injection. Peristimulus rate histograms (5-min bins) were normalized by dividing the postinjection firing rates by the baseline firing rate. (A) Neurons with type 1 responses had a sustained increase in firing rate for high doses of MK801. (B) Neurons with type 2 responses were not significantly different from saline. (C) MK801 at moderate and high doses significantly increased stereotypy scores.
Fig. 3.
Comparison of firing rate changes and behavioral changes after systemic MK801 injection. (A) The mean area under the normalized postinjection rate histogram for type 1 neurons in each group is plotted on the left vertical axis. The area gives an indication of the total neural activity during this period. Total stereotypy score is plotted on the right vertical axis. Increases in neural activity at the two highest doses of MK801 are highly correlated with increases in stereotypy. (B) Plot of correlation between firing rate increases and stereotypy scores for individual experiments. Stereotypy scores recorded in 5-min time bins were correlated with the averaged rate histogram (5-min bins) of all type 1 neurons recorded in that same animal. There were poor correlations between behavior and firing rate for saline and MK801 at 0.01 mg/kg and 0.05 mg/kg, but high correlations at the two higher doses of MK801. Asterisks indicate significant difference compared to saline by ANOVA with the Bonferroni post hoc test (P < 0.05).
Fig. 4.
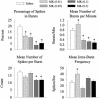
Changes in burst parameters after MK801 injection. For neurons that had an increase in firing rate after MK801 injection, there was a dose-dependent decrease in the percentage of spikes in bursts, mean number of bursts per min, and mean number of spikes per burst. However, for neurons in the 0.01 mg/kg MK801 group, there was a significant increase in the mean number of bursts per min and mean intraburst frequency. Asterisks indicate significant difference compared to saline by ANOVA with the Bonferroni post hoc test (P < 0.05).
Fig. 5.
The percentage of spikes contained within bursts decreased with high doses of MK801. The percentage of spikes contained within bursts was calculated for each neuron during a 30-min baseline period and during four sequential 30-min postinjection periods. Data for all groups and response types is summarized by plotting the linear regression coefficients. Numbers under each bar indicate the postinjection time period as follows: lanes 1, 0–30 min; lanes 2, 30–60 min; lanes 3, 60–90 min; and lanes 4, 90–120 min. Higher doses of MK801 caused a significant decrease in the percentage of neurons in bursts, regardless of the response type. Asterisks indicate significant difference from a regression coefficient of 1.0, which indicates no change from baseline.
Fig. 6.
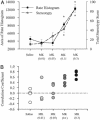
Effect of MK801 on spontaneous alternation performance. (A) There was a differential dose-dependent effect of MK801 on percent alternation. The lowest dose of MK801 (0.01 mg/kg) improved performance, whereas higher doses (0.5 and 0.1 mg/kg) impaired performance. Asterisk indicates significant difference by ANOVA with the Bonferroni post hoc test (P < 0.05).
Similar articles
- Activation of metabotropic glutamate 2/3 receptors reverses the effects of NMDA receptor hypofunction on prefrontal cortex unit activity in awake rats.
Homayoun H, Jackson ME, Moghaddam B. Homayoun H, et al. J Neurophysiol. 2005 Apr;93(4):1989-2001. doi: 10.1152/jn.00875.2004. Epub 2004 Dec 8. J Neurophysiol. 2005. PMID: 15590730 - AMPA and NMDA receptor regulation of firing activity in 5-HT neurons of the dorsal and median raphe nuclei.
Gartside SE, Cole AJ, Williams AP, McQuade R, Judge SJ. Gartside SE, et al. Eur J Neurosci. 2007 May;25(10):3001-8. doi: 10.1111/j.1460-9568.2007.05577.x. Epub 2007 May 17. Eur J Neurosci. 2007. PMID: 17509083 - The effect of non-competitive NMDA receptor antagonist MK-801 on neuronal activity in rodent prefrontal cortex: an animal model for cognitive symptoms of schizophrenia.
Blot K, Bai J, Otani S. Blot K, et al. J Physiol Paris. 2013 Dec;107(6):448-51. doi: 10.1016/j.jphysparis.2013.04.003. Epub 2013 Apr 18. J Physiol Paris. 2013. PMID: 23603055 Review. - [Molecular pathology of schizophrenia].
Uezato A, Nishikawa T. Uezato A, et al. Nihon Rinsho. 2013 Apr;71(4):591-8. Nihon Rinsho. 2013. PMID: 23678584 Review. Japanese.
Cited by
- Memories reactivated under ketamine are subsequently stronger: A potential pre-clinical behavioral model of psychosis.
Honsberger MJ, Taylor JR, Corlett PR. Honsberger MJ, et al. Schizophr Res. 2015 May;164(1-3):227-33. doi: 10.1016/j.schres.2015.02.009. Epub 2015 Feb 24. Schizophr Res. 2015. PMID: 25728834 Free PMC article. - Beneficial effects of the NMDA antagonist ketamine on decision processes in visual search.
Shen K, Kalwarowsky S, Clarence W, Brunamonti E, Paré M. Shen K, et al. J Neurosci. 2010 Jul 21;30(29):9947-53. doi: 10.1523/JNEUROSCI.6317-09.2010. J Neurosci. 2010. PMID: 20660277 Free PMC article. - Shared and Distinct Brain Regions Targeted for Immediate Early Gene Expression by Ketamine and Psilocybin.
Davoudian PA, Shao LX, Kwan AC. Davoudian PA, et al. ACS Chem Neurosci. 2023 Feb 1;14(3):468-480. doi: 10.1021/acschemneuro.2c00637. Epub 2023 Jan 11. ACS Chem Neurosci. 2023. PMID: 36630309 Free PMC article. - meaRtools: An R package for the analysis of neuronal networks recorded on microelectrode arrays.
Gelfman S, Wang Q, Lu YF, Hall D, Bostick CD, Dhindsa R, Halvorsen M, McSweeney KM, Cotterill E, Edinburgh T, Beaumont MA, Frankel WN, Petrovski S, Allen AS, Boland MJ, Goldstein DB, Eglen SJ. Gelfman S, et al. PLoS Comput Biol. 2018 Oct 1;14(10):e1006506. doi: 10.1371/journal.pcbi.1006506. eCollection 2018 Oct. PLoS Comput Biol. 2018. PMID: 30273353 Free PMC article. - The impact of D-cycloserine and sarcosine on in vivo frontal neural activity in a schizophrenia-like model.
Yao L, Wang Z, Deng D, Yan R, Ju J, Zhou Q. Yao L, et al. BMC Psychiatry. 2019 Oct 25;19(1):314. doi: 10.1186/s12888-019-2306-1. BMC Psychiatry. 2019. PMID: 31653237 Free PMC article.
References
- Harrison, P. J. & Owen, M. J. (2003) Lancet 361, 417-419. - PubMed
- Moghaddam, B. (2003) Neuron 40, 881-884. - PubMed
- Krystal, J. H., Karper, L. P., Seibyl, J. P., Freeman, G. K., Delaney, R., Bremner, J. D., Heninger, G. R., Bowers, M., Jr., & Charney, D. S. (1994) Arch. Gen. Psychiatry 51, 199-214. - PubMed
- Lahti, A. C., Koffel, B., LaPorte, D. & Tamminga, C. A. (1995) Neuropsychopharmacology 13, 9-19. - PubMed
- Newcomer, J. W., Farber, N. B., Jevtovic-Todorovic, V., Selke, G., Melson, A. K., Hershey, T., Craft, S. & Olney, J. W. (1999) Neuropsychopharmacology 20, 106-118. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical