Leukocyte-derived interleukin-1beta interacts with renal interleukin-1 receptor I to promote renal tumor necrosis factor and glomerular injury in murine crescentic glomerulonephritis - PubMed (original) (raw)
Leukocyte-derived interleukin-1beta interacts with renal interleukin-1 receptor I to promote renal tumor necrosis factor and glomerular injury in murine crescentic glomerulonephritis
Jennifer R Timoshanko et al. Am J Pathol. 2004 Jun.
Abstract
The involvement of proinflammatory cytokines interleukin (IL)-1 and tumor necrosis factor (TNF) in crescentic glomerulonephritis (GN) is well established. Recently the requirement of intrinsic renal cell participation via their production of TNF in crescentic GN was demonstrated. The current studies address the relative contributions of leukocyte and intrinsic renal cell-derived IL-1beta in the induction of TNF production and glomerular injury by studying bone marrow chimeric mice. Leukocyte-derived IL-1beta was critical in the development of crescentic renal injury because IL-1beta(-/-)-->WT (absent leukocyte IL-1beta) chimeric mice had significantly attenuated TNF expression and were protected from the development of crescentic GN. In contrast, WT-->IL-1beta(-/-) chimeric mice (intact leukocyte but absent renal IL-1beta) developed similar TNF expression and crescentic GN to wild-type mice. To determine the cellular target for IL-1 in this model, IL-RI chimeric mice were studied. IL-1RI(-/-)-->WT chimeric (absent leukocyte IL-1RI expression) mice showed no attenuation of crescentic GN, whereas in the absence of renal IL-1RI (WT-->IL-1RI(-/-) chimeras), glomerular TNF expression and the development of crescentic GN were significantly decreased. These studies demonstrate that leukocytes are the major cellular source of IL-1beta, and that IL-1beta acts principally via the IL-1RI on intrinsic renal cells to induce TNF expression and crescentic glomerular injury.
Figures
Figure 1
Renal localization of IL-β (green, A–E) and IL-1RI (red, F–J) in WT and chimeric mice with GN, detected by immunofluorescence, and captured by confocal microscopy. A: In glomeruli of WT mice, cytoplasmic staining of infiltrating leukocytes with occasional patchy staining of resident glomerular cells was observed. Tubular areas were also positive for IL-1β. B: In WT→IL-1β−/− chimeras IL-1β was only detected on macrophages infiltrating the kidney, no renal expression was detected. C: In IL-1β−/−→WT chimeric animals, IL-1β expression was sparsely observed in glomeruli and on tubular cells. In IL-1RI chimeric mice, WT→IL-1RI−/− (D), and IL-1RI−/−→WT (E), IL-1β expression was observed in a similar pattern to WT animals. Renal expression of IL-1RI in WT (F), WT→IL-1β−/− (G), and IL-1β−/−→WT (H) mice was similar, with the receptor observed to be present on cells in the glomerulus and on tubules. I: In contrast the WT→IL-1RI−/− chimeras had absent renal IL-1RI expression with limited expression detected on infiltrating inflammatory cells. J: The expression of IL-1RI in IL-1RI−/−→WT chimeras was not noticeably reduced compared with WT mice, this indicating that the prominent expression observed in WT and IL-1β chimeric mice to be by intrinsic renal cells. Confocal immunofluorescence, under oil immersion. Original magnifications, ×600.
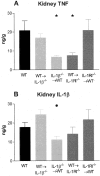
Figure 2
Renal TNF and IL-1β levels were measured in WT and chimeric mice with GN. Kidney samples were homogenized and protein extracted, results expressed as concentration (ng) per tissue wet weight (g). A: Kidney TNF expression was significantly reduced in IL-1β−/−→WT and WT→IL-1RI−/− chimeric mice compared to WT mice (*, P < 0.05). B: Renal IL-1β expression was reduced in IL-1β−/−→WT chimeras compared to WT→IL-1β−/− chimeras (•, P < 0.05).
Figure 3
The histological appearance of crescentic glomerular injury in WT and chimeric mice 21 days after administration of sheep anti-mouse GBM globulin. A: WT mice developed proliferative GN with glomerular crescent formation, deposition of glomerular periodic acid-Schiff-positive material and cellular interstitial infiltrate. Proliferative changes and crescents were also observed in WT→IL-1β−/− chimeric (B) and IL-1RI−/−→WT chimeric (E) mice with GN. IL-1β−/−→WT chimeric (C) and WT→IL-1RI−/− chimeric (D) mice developed mild proliferative GN, with only occasionally observed crescent formation. Periodic acid-Schiff-stained sections. Original magnifications, ×400.
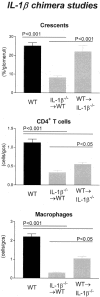
Figure 4
Histological features of glomerular injury in WT and IL-1β chimeric mice with GN: glomerular crescent formation, glomerular CD4+ T cell accumulation, and glomerular macrophage accumulation. Control WT mice with GN developed severe GN with a high incidence of glomerular crescent formation. In contrast IL-1β−/−→WT chimeric mice had a significant reduction in the proportion of glomeruli affected. WT→IL-1β−/− chimeric mice had a similar incidence of crescent formation to WT controls, which was significantly higher than IL-1β−/−→WT chimeric mice. Glomerular accumulation of CD4+ T cells and macrophages was significantly reduced in both chimeric groups, with a trend to a greater reduction observed in IL-1β−/−→WT chimeras because inflammatory cellular infiltrate is significantly reduced compared to WT→IL-1β−/− chimeras.
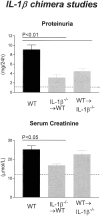
Figure 5
Functional indices of injury in IL-1β chimeric mice with GN. WT mice with GN developed significant proteinuria, whereas IL-1β chimeric mice had reduced proteinuria compared with WT mice. Elevated serum creatinine demonstrated impaired renal function in WT and WT→IL-β−/− chimeric mice, whereas significantly lower serum creatinine levels were demonstrated in IL-1β−/−→WT chimeric mice. The dotted line indicates the urinary protein excretion and serum creatinine of normal WT mice without GN.
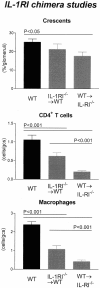
Figure 6
Histological features of glomerular injury in WT and IL-1RI chimeric mice with GN: glomerular crescent formation, glomerular CD4+ T cell accumulation, and glomerular macrophage accumulation. Control WT mice with GN developed severe GN with a high incidence of glomerular crescent formation. Similarly WT→IL-1RI−/− chimeric mice formed numerous crescents with a high incidence of glomeruli affected. In contrast WT→IL-1RI−/− chimeric mice had a significant reduction in the proportion of crescentic glomeruli compared to WT mice. Glomerular accumulation of CD4+ T cells and macrophages was significantly reduced in both chimeric groups, with a trend to a greater reduction observed in WT→IL-1RI−/− chimeras because inflammatory cellular infiltrate is significantly reduced compared to IL-1RI−/−→WT chimeras.
Figure 7
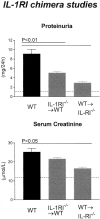
Functional indices of injury in IL-1RI chimeric mice with GN. IL-1RI chimeric mice developed significantly less proteinuria compared with WT mice with GN. Serum creatinine demonstrated impaired renal function in WT and IL-1RI−/−→WT chimeric mice, whereas WT→IL-1RI−/− chimeric mice had significantly lower serum creatinine than WT mice. The dotted line indicates the urinary protein excretion and serum creatinine of normal WT mice without GN.
Figure 8
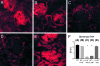
Immunofluorescence assessment of renal TNF expression. A: In WT mice, prominent glomerular TNF was observed on intraglomerular cells, particularly in the glomerular stalk. TNF expression was also observed on tubular cells. This prominent pattern of TNF expression was also observed in WT→IL-1β−/− (B) and IL-1RI−/−→WT (E) chimeras. In contrast, TNF expression was significantly reduced in IL-1β−/−→WT (C) and WT→IL-RI−/− (D) chimeric mice, with minimal TNF expression on intraglomerular cells and in interstitial area. F: Semiquantitative analysis showed significant reduction in renal TNF expression in IL-1β−/−→WT and IL-1RI−/−→WT chimeras compared to other groups (▪, P < 0.001; see WT) Confocal immunofluorescence, under oil immersion. Original magnifications, ×600.
Figure 9
Circulating mouse anti-sheep globulin titers in chimeric mice with crescentic GN demonstrating similar levels of serum anti-sheep globulin antibody in WT, IL-1β−/−→WT, and WT→IL-1β−/− groups. Nonsignificant reductions of serum anti-sheep globulin antibody titers in WT→IL-1RI−/− and IL-1RI−/−→WT chimeric groups were observed. Bottom dotted line represents the circulating serum anti-sheep globulin antibody levels observed in normal WT mice without GN.
Similar articles
- Granulocyte macrophage colony-stimulating factor expression by both renal parenchymal and immune cells mediates murine crescentic glomerulonephritis.
Timoshanko JR, Kitching AR, Semple TJ, Holdsworth SR, Tipping PG. Timoshanko JR, et al. J Am Soc Nephrol. 2005 Sep;16(9):2646-56. doi: 10.1681/ASN.2004121107. Epub 2005 Jul 20. J Am Soc Nephrol. 2005. PMID: 16033860 - Intrinsic renal cells are the major source of tumor necrosis factor contributing to renal injury in murine crescentic glomerulonephritis.
Timoshanko JR, Sedgwick JD, Holdsworth SR, Tipping PG. Timoshanko JR, et al. J Am Soc Nephrol. 2003 Jul;14(7):1785-93. doi: 10.1097/01.asn.0000073902.38428.33. J Am Soc Nephrol. 2003. PMID: 12819238 - Interleukin-12 from intrinsic cells is an effector of renal injury in crescentic glomerulonephritis.
Timoshanko JR, Kitching AR, Holdsworth SR, Tipping PG. Timoshanko JR, et al. J Am Soc Nephrol. 2001 Mar;12(3):464-471. doi: 10.1681/ASN.V123464. J Am Soc Nephrol. 2001. PMID: 11181794 - Contributions of intrinsic renal cells to crescentic glomerulonephritis.
Tipping PG, Timoshanko J. Tipping PG, et al. Nephron Exp Nephrol. 2005;101(4):e173-8. doi: 10.1159/000088165. Epub 2005 Sep 8. Nephron Exp Nephrol. 2005. PMID: 16155400 Review. - Resident kidney cells and their involvement in glomerulonephritis.
Timoshanko JR, Tipping PG. Timoshanko JR, et al. Curr Drug Targets Inflamm Allergy. 2005 Jun;4(3):353-62. doi: 10.2174/1568010054022132. Curr Drug Targets Inflamm Allergy. 2005. PMID: 16101545 Review.
Cited by
- Distinct contributions of TNF receptor 1 and 2 to TNF-induced glomerular inflammation in mice.
Taubitz A, Schwarz M, Eltrich N, Lindenmeyer MT, Vielhauer V. Taubitz A, et al. PLoS One. 2013 Jul 15;8(7):e68167. doi: 10.1371/journal.pone.0068167. Print 2013. PLoS One. 2013. PMID: 23869211 Free PMC article. - Predictive value of conjointly examined IL-1ra, TNF-R I, TNF-R II, and RANTES in patients with primary glomerulonephritis.
Zwiech R. Zwiech R. J Korean Med Sci. 2013 Feb;28(2):261-7. doi: 10.3346/jkms.2013.28.2.261. Epub 2013 Jan 29. J Korean Med Sci. 2013. PMID: 23400706 Free PMC article. - ATRvD1 Attenuates Renal Tubulointerstitial Injury Induced by Albumin Overload in Sepsis-Surviving Mice.
Silva JBNF, Calcia TBB, Silva CP, Guilherme RF, Almeida-Souza F, Lemos FS, Calabrese KS, Caruso-Neves C, Neves JS, Benjamim CF. Silva JBNF, et al. Int J Mol Sci. 2021 Oct 27;22(21):11634. doi: 10.3390/ijms222111634. Int J Mol Sci. 2021. PMID: 34769064 Free PMC article. - Interleukin-1 cluster gene polymorphisms in childhood IgA nephropathy.
Hahn WH, Cho BS, Kim SD, Kim SK, Kang S. Hahn WH, et al. Pediatr Nephrol. 2009 Jul;24(7):1329-36. doi: 10.1007/s00467-009-1146-5. Epub 2009 Mar 12. Pediatr Nephrol. 2009. PMID: 19280228 - Interleukin-1β suppresses activity of an inwardly rectifying K+ channel in human renal proximal tubule cells.
Nakamura K, Komagiri Y, Kubokawa M. Nakamura K, et al. J Physiol Sci. 2013 Sep;63(5):377-87. doi: 10.1007/s12576-013-0275-6. J Physiol Sci. 2013. PMID: 23797607 Free PMC article.
References
- Holdsworth SR, Kitching AR, Tipping PG. Th1 and Th2 T helper cell subsets affect patterns of injury and outcomes in glomerulonephritis. Kidney Int. 1999;55:1198–1216. - PubMed
- Huang XR, Holdsworth SR, Tipping PG. Evidence for delayed-type hypersensitivity mechanisms in glomerular crescent formation. Kidney Int. 1994;46:69–78. - PubMed
- Huang XR, Tipping PG, Shuo L, Holdsworth SR. Th1 responsiveness to nephritogenic antigens determines susceptibility to crescentic glomerulonephritis in mice. Kidney Int. 1997;51:94–103. - PubMed
- Kitching AR, Tipping PG, Holdsworth SR. IL-12 directs severe renal injury, crescent formation and Th1 responses in murine glomerulonephritis. Eur J Immunol. 1999;29:1–10. - PubMed
- Kitching AR, Holdsworth SR, Tipping PG. IFN-gamma mediates crescent formation and cell-mediated immune injury in murine glomerulonephritis. J Am Soc Nephrol. 1999;10:752–759. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases