Activation of Stat3 transcription factor by Herpesvirus saimiri STP-A oncoprotein - PubMed (original) (raw)
Activation of Stat3 transcription factor by Herpesvirus saimiri STP-A oncoprotein
Young-Hwa Chung et al. J Virol. 2004 Jun.
Abstract
The saimiri transforming protein (STP) oncogene of Herpesvirus saimiri subgroup A strain 11 (STP-A11) is not required for viral replication but is required for lymphoid cell immortalization in culture and lymphoma induction in primates. We previously showed that STP-A11 interacts with cellular Src kinase through its SH2 binding motif and that this interaction elicits Src signal transduction. Here we demonstrate that STP-A11 interacts with signal transducer and activator of transcription 3 (Stat3) independently of Src association and that the amino-terminal short proline-rich motif of STP-A11 and the central linker region of Stat3 are necessary for their interaction. STP-A11 formed a triple complex with Src kinase and Stat3 where Src kinase phosphorylated Stat3, resulting in the nuclear localization and transcriptional activation of Stat3. Consequently, the constitutively active Stat3 induced by STP-A11 elicited cellular signal transduction, which ultimately induced cell survival and proliferation upon serum deprivation. Furthermore, this activity was strongly correlated with the induction of Fos, cyclin D1, and Bcl-XL expression. These results demonstrate that STP-A11 independently targets two important cellular signaling molecules, Src and Stat3, and that these proteins cooperate efficiently to induce STP-A11-mediated transformation.
Figures
FIG. 1.
Activation of Stat transcription activity by STP-A11 and Src expression. 293T cells were transfected with a Stat3 luciferase reporter vector (pLuc-TKS3) and an expression vector containing either wt STP-A11 or the Y115A mutant either alone or together with a Src expression vector. At 48 h posttransfection, cells were harvested, and luciferase activity was measured. Transfection efficiency was normalized with the β-galactosidase reporter vector pGK-βgal. Results are averages from three independent experiments. Error bars, standard deviations. Gels at the bottom show the expression of STP-A11, its mutant, and Src in whole-cell lysates.
FIG. 2.
Specific interaction of STP-A11 with Stat3. (A) STP-A11 interacts with Stat3 but not with Stat1 or Stat2. At 48 h after transfection with the carboxy-terminal HA-tagged STP-A11 expression vector alone (lanes 1 and 2) or together with the Stat3 expression vector (lane 3), 293T cells were lysed and used for immunoprecipitation (IP) with an anti-HA (αHA)antibody, followed by immunoblotting with an anti-Stat1, anti-Stat2, or anti-Stat3 antibody. To demonstrate the expression of endogenous Stat1 and Stat2 and transfected Stat3, whole-cell lysates (WCL) were used for immunoblotting with an anti-Stat1, anti-Stat2, or anti-Stat3 antibody and an anti-HA antibody. (B) Stat3 interacts with STP-A11 and is phosphorylated at Y705 by Src kinase associated with STP-A11. At 48 h after transfection with various expression vectors, 293T cells were lysed and used for immunoprecipitation with an anti-HA antibody, followed by immunoblotting with an anti-Stat3 antibody, an anti-Stat3 Y705 phosphospecific antibody, or an anti-Src antibody. Lane 1, vector only; lane 2, Stat3; lane 3, STP-A11; lane 4, Stat3 plus STP-A11; lane 5, Stat3 plus STP-A11 Y115; lane 6, Stat3 plus STP-A11 plus Src; lane 7, Stat3Y705A plus STP-A11 plus Src.
FIG. 3.
Identification of the regions of Stat3 and STP-A11 necessary for their interaction. (A) The central linker region of Stat3 is sufficient for interacting with STP-A11. Bacterially purified GST or GST fusion proteins containing the amino-terminal region (ΔCST3), the central linker region, or the carboxyl-terminal region (ΔNST3) of Stat3 were mixed with lysates of 293T cells transfected with the HA-tagged STP-A11 expression vector. Polypeptides present in GST beads were used for immunoblotting (IB) with an anti-HA (α-HA) antibody. Similar amounts of each GST fusion protein were used in this assay (data not shown). ND, N-terminal domain; TAD, transcriptional activation domain. (B) The amino-terminal region of STP-A11 is necessary for interacting with Stat3. Bacterially purified GST or GST fusion proteins containing the amino-terminal regions of STP-A11 were mixed with lysates of 293T cells transfected with the Stat3 expression vector. Polypeptides present in GST beads were used for immunoblotting with an anti-Stat3 antibody. Similar amounts of each GST fusion protein were used in this assay (data not shown). TM, transmembrane domain. (C) The amino-terminal proline-rich motif of STP-A11 is necessary for interacting with Stat3. At 48 h after transfection with a Stat3 expression vector together with an expression vector containing the HA-tagged wt STP-A11 or its mutant, 293T cells were lysed and used for immunoprecipitation with an anti-HA antibody, followed by immunoblotting with an anti-Stat3 antibody.
FIG. 4.
Effect of the interaction of STP-A11 with Stat3 and Src on the efficient activation of Stat3 transcriptional activity. 293T cells were transfected with a Stat3 luciferase reporter vector (pLuc-TKS3) and an expression vector containing wt STP-A11 or the Y115A, D1, D2, D3, or Y115A/D1 mutant in the presence or absence of the Src expression vector. At 48 h posttransfection, cells were harvested for measurement of luciferase activity. Transfection efficiency was normalized with the β-galactosidase reporter vector pGK-βgal. Results are averages from three independent experiments. Error bars, standard deviations. Gels at the bottom show expression of STP-A11, its mutants, and Src in whole-cell lysates.
FIG. 5.
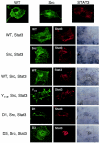
Nuclear localization of Stat3 upon STP-A11 and Src expression. Cos-1 cells were transfected with a Stat3 expression vector alone or together with pBud expression vectors carrying c-Src and/or wt or mutant STP-A11. At 48 h posttransfection, cells were fixed and reacted with anti-Stat3 (red), anti-STP-A11 (green), and/or anti-Src (green) antibodies, followed by incubation with an Alexa-488-conjugated anti-mouse immunoglobulin G antibody and/or an Alexa-568-conjugated anti-rabbit immunoglobulin G antibody. Immunofluorescence was examined with a Leica confocal microscope. Cells were visualized with Nomarski optics.
FIG. 6.
Contribution of STP-A11-induced Stat3 activation to enhanced cell growth. (A) Expression of wt STP-A and its mutants in stable NIH 3T3 cells. Lysates from NIH 3T3-puro (Vec), NIH 3T3-wt STP-A11 (WT), NIH 3T3-STP-A11 Y115A (Y115), and NIH 3T3-STP-A11 D1 (D1) cells were prepared and blotted with an anti-AU1 antibody for detection of STP-A. Tubulin was used for loading equivalent amount of proteins from each sample. (B) Cell growth rate upon serum deprivation. NIH 3T3-puro, NIH 3T3-wt STP-A11, NIH 3T3-STP-A11 Y115A, and NIH 3T3-STP-A11 D1 cells were incubated with 0.1, 0.2, or 1% serum. Cell proliferation rates of these NIH 3T3 cells were measured by a modified MTT assay. Results are averages from three independent experiments. Error bars, standard deviations. (C) Y705 phosphorylation of Stat3 and enhanced expression of Fos, cyclin D1, and Bcl-XL upon STP-A11 expression. Equivalent amounts of polypeptides from NIH 3T3-puro, NIH 3T3-wt STP-A11, NIH 3T3-STP-A11 Y115A, and NIH 3T3-STP-A11 D1 cells were used for immunoblotting with an anti-Stat3 antibody, an anti-Stat3 Y705 phosphospecific antibody, an anti-Fos antibody, an anti-cyclin D1 antibody, or an anti-Bcl-XL antibody.
Similar articles
- Herpesvirus saimiri STP A11 protein interacts with STAT3 and stimulates its transcriptional activity.
Park J, Seo T, Jung J, Choe J. Park J, et al. Biochem Biophys Res Commun. 2004 Jul 16;320(1):279-85. doi: 10.1016/j.bbrc.2004.05.162. Biochem Biophys Res Commun. 2004. PMID: 15207733 - STP-A11, an oncoprotein of Herpesvirus saimiri augments both NF-kappaB and AP-1 transcription activity through TRAF6.
Jeong S, Cho IR, An WG, Jhun BH, Lee B, Park K, Chung YH. Jeong S, et al. Exp Mol Med. 2007 Feb 28;39(1):56-64. doi: 10.1038/emm.2007.7. Exp Mol Med. 2007. PMID: 17334229 - Herpesvirus saimiri STP-A oncoprotein utilizes Src family protein tyrosine kinase and tumor necrosis factor receptor-associated factors to elicit cellular signal transduction.
Garcia MI, Kaserman J, Chung YH, Jung JU, Lee SH. Garcia MI, et al. J Virol. 2007 Mar;81(6):2663-74. doi: 10.1128/JVI.01733-06. Epub 2006 Dec 20. J Virol. 2007. PMID: 17182673 Free PMC article. - Genetic variation of herpesvirus saimiri subgroup A transforming protein and its association with cellular src.
Lee H, Trimble JJ, Yoon DW, Regier D, Desrosiers RC, Jung JU. Lee H, et al. J Virol. 1997 May;71(5):3817-25. doi: 10.1128/JVI.71.5.3817-3825.1997. J Virol. 1997. PMID: 9094657 Free PMC article. - Herpesvirus saimiri.
Fickenscher H, Fleckenstein B. Fickenscher H, et al. Philos Trans R Soc Lond B Biol Sci. 2001 Apr 29;356(1408):545-67. doi: 10.1098/rstb.2000.0780. Philos Trans R Soc Lond B Biol Sci. 2001. PMID: 11313011 Free PMC article. Review.
Cited by
- A Central Role for STAT3 in Gammaherpesvirus-Life Cycle and -Diseases.
Li X, Bhaduri-McIntosh S. Li X, et al. Front Microbiol. 2016 Jul 8;7:1052. doi: 10.3389/fmicb.2016.01052. eCollection 2016. Front Microbiol. 2016. PMID: 27458446 Free PMC article. Review. - Tyrosine phosphorylation of the Tio oncoprotein is essential for transformation of primary human T cells.
Albrecht JC, Müller-Fleckenstein I, Schmidt M, Fleckenstein B, Biesinger B. Albrecht JC, et al. J Virol. 2005 Aug;79(16):10507-13. doi: 10.1128/JVI.79.16.10507-10513.2005. J Virol. 2005. PMID: 16051843 Free PMC article. - A molecular model for the differential activation of STAT3 and STAT6 by the herpesviral oncoprotein tip.
Mazumder ED, Jardin C, Vogel B, Heck E, Scholz B, Lengenfelder D, Sticht H, Ensser A. Mazumder ED, et al. PLoS One. 2012;7(4):e34306. doi: 10.1371/journal.pone.0034306. Epub 2012 Apr 3. PLoS One. 2012. PMID: 22509288 Free PMC article. - The Complex Role of STAT3 in Viral Infections.
Kuchipudi SV. Kuchipudi SV. J Immunol Res. 2015;2015:272359. doi: 10.1155/2015/272359. Epub 2015 Jun 25. J Immunol Res. 2015. PMID: 26199948 Free PMC article. Review. - ERp57 modulates STAT3 signaling from the lumen of the endoplasmic reticulum.
Coe H, Jung J, Groenendyk J, Prins D, Michalak M. Coe H, et al. J Biol Chem. 2010 Feb 26;285(9):6725-38. doi: 10.1074/jbc.M109.054015. Epub 2009 Dec 18. J Biol Chem. 2010. PMID: 20022947 Free PMC article.
References
- Biesinger, B., J. J. Trimble, R. C. Desrosiers, and B. Fleckenstein. 1990. The divergence between two oncogenic Herpesvirus saimiri strains in a genomic region related to the transforming phenotype. Virology 176:505-514. - PubMed
- Bromberg, J. F., M. H. Wrzeszczynska, G. Devgan, Y. Zhao, R. G. Pestell, C. Albanese, and J. E. Darnell, Jr. 1999. Stat3 as an oncogene. Cell 98:295-303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RR 00168/RR/NCRR NIH HHS/United States
- P51 RR000168/RR/NCRR NIH HHS/United States
- K26 RR000168/RR/NCRR NIH HHS/United States
- AI 38131/AI/NIAID NIH HHS/United States
- CA 31363/CA/NCI NIH HHS/United States
- R01 CA031363/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous