Inflammatory cells contribute to the generation of an angiogenic phenotype in pancreatic ductal adenocarcinoma - PubMed (original) (raw)
Inflammatory cells contribute to the generation of an angiogenic phenotype in pancreatic ductal adenocarcinoma
I Esposito et al. J Clin Pathol. 2004 Jun.
Abstract
Background: Inflammatory cells contribute to the growth and spread of human malignancies by producing molecules that enhance tumour invasiveness.
Aims: To characterise the inflammatory infiltrate in pancreatic ductal adenocarcinoma and to analyse its contribution to angiogenesis and its prognostic relevance.
Methods: Immunohistochemistry was used to identify inflammatory cells and evaluate the expression of proangiogenic and prolymphangiogenic molecules (vascular endothelial growth factor A (VEGF-A), VEGF-C, and basic fibroblast growth factor (bFGF)) by inflammatory and cancer cells in 137 pancreatic cancers. Intratumorous microvessel density (IMD) was assessed using CD34 as an endothelial cell marker.
Results: There were significantly more mast cells and macrophages in pancreatic cancers than in normal pancreas and the number of mast cells directly correlated with the presence of lymph node metastases. However, there was no relation between numbers of infiltrating inflammatory cells and the presence of chronic pancreatitis (CP)-like changes in the parenchyma surrounding the tumour. Double immunostaining revealed that both pancreatic mast cells and macrophages express VEGF-A, VEGF-C, and bFGF. These factors were also expressed in the tumour cells in many cases. The numbers of VEGF-A expressing tumour cells and bFGF expressing tumour and inflammatory cells significantly correlated with IMD. Moreover, tumours with higher IMD had higher numbers of infiltrating mast cells and macrophages.
Conclusions: Mononuclear inflammatory cells of the non-specific immune response are recruited to pancreatic cancer tissues independent of the presence of CP-like changes, may influence the metastatic capacity of the cancer cells, and may contribute to the development of tumours with high angiogenic activity.
Figures
Figure 1
Expression of proangiogenic growth factors in pancreatic ductal adenocarcinoma. Strong immunoreactivity for (A) vascular endothelial growth factor A (VEGF-A), (B) VEGF-C, and (C) basic fibroblast growth factor in the cytoplasm of the tumour cells; positive inflammatory cells are visible in the stroma around the tumour cells and (D) at the tumour periphery.
Figure 2
Characterisation of the inflammatory infiltrate in pancreatic ductal adenocarcinoma. (A) Tryptase positive mast cells and (B) CD68 positive macrophages are the most common inflammatory cells in the stroma of pancreatic ductal adenocarcinoma. Double immunohistochemistry shows that (C) mast cells and (D) macrophages (red staining) are a source of vascular endothelial growth factor C (brown staining) in pancreatic ductal adenocarcinoma. The arrowheads point to double positive inflammatory cells, the arrows to single (brown) stained inflammatory cells.
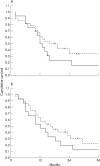
Figure 3
Survival analysis. (A) The survival of patients whose tumours exhibited a high number of infiltrating mast cells and a high ntratumorous microvessel density (IMD; solid line) was shorter than that of patients whose tumours exhibited a low number of infiltrating mast cells and a low IMD (broken line). However, the difference was not significant. (B) The same tendency was seen when the number of infiltrating macrophages was combined with the IMD (solid line, high number of macrophages and high IMD; broken line, low number of macrophages and low IMD). Crosses indicate censored cases.
Similar articles
- Angiogenesis in pancreatic carcinoma: thymidine phosphorylase expression in stromal cells and intratumoral microvessel density as independent predictors of overall and relapse-free survival.
Fujioka S, Yoshida K, Yanagisawa S, Kawakami M, Aoki T, Yamazaki Y. Fujioka S, et al. Cancer. 2001 Oct 1;92(7):1788-97. doi: 10.1002/1097-0142(20011001)92:7<1788::aid-cncr1695>3.0.co;2-z. Cancer. 2001. PMID: 11745251 - VEGF and Id-1 in pancreatic adenocarcinoma: prognostic significance and impact on angiogenesis.
Georgiadou D, Sergentanis TN, Sakellariou S, Filippakis GM, Zagouri F, Vlachodimitropoulos D, Psaltopoulou T, Lazaris AC, Patsouris E, Zografos GC. Georgiadou D, et al. Eur J Surg Oncol. 2014 Oct;40(10):1331-7. doi: 10.1016/j.ejso.2014.01.004. Epub 2014 Jan 18. Eur J Surg Oncol. 2014. PMID: 24480377 - Expression of hypoxia-inducible factors is correlated with the presence of a fibrotic focus and angiogenesis in pancreatic ductal adenocarcinomas.
Couvelard A, O'Toole D, Leek R, Turley H, Sauvanet A, Degott C, Ruszniewski P, Belghiti J, Harris AL, Gatter K, Pezzella F. Couvelard A, et al. Histopathology. 2005 Jun;46(6):668-76. doi: 10.1111/j.1365-2559.2005.02160.x. Histopathology. 2005. PMID: 15910598 - Mast cells and angiogenesis in pancreatic ductal adenocarcinoma.
Longo V, Tamma R, Brunetti O, Pisconti S, Argentiero A, Silvestris N, Ribatti D. Longo V, et al. Clin Exp Med. 2018 Aug;18(3):319-323. doi: 10.1007/s10238-018-0493-6. Epub 2018 Feb 28. Clin Exp Med. 2018. PMID: 29492715 Review. - Overview of pre-clinical and clinical studies targeting angiogenesis in pancreatic ductal adenocarcinoma.
Craven KE, Gore J, Korc M. Craven KE, et al. Cancer Lett. 2016 Oct 10;381(1):201-10. doi: 10.1016/j.canlet.2015.11.047. Epub 2015 Dec 23. Cancer Lett. 2016. PMID: 26723874 Free PMC article. Review.
Cited by
- Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma.
Zheng L, Xue J, Jaffee EM, Habtezion A. Zheng L, et al. Gastroenterology. 2013 Jun;144(6):1230-40. doi: 10.1053/j.gastro.2012.12.042. Gastroenterology. 2013. PMID: 23622132 Free PMC article. Review. - Pilot study to relate clinical outcome in pancreatic carcinoma and angiogenic plasma factors/circulating mature/progenitor endothelial cells: Preliminary results.
Vizio B, Novarino A, Giacobino A, Cristiano C, Prati A, Brondino G, Ciuffreda L, Bellone G. Vizio B, et al. Cancer Sci. 2010 Nov;101(11):2448-54. doi: 10.1111/j.1349-7006.2010.01692.x. Cancer Sci. 2010. PMID: 20950371 Free PMC article. - Mast Cells and Natural Killer Cells-A Potentially Critical Interaction.
Portales-Cervantes L, Dawod B, Marshall JS. Portales-Cervantes L, et al. Viruses. 2019 Jun 4;11(6):514. doi: 10.3390/v11060514. Viruses. 2019. PMID: 31167464 Free PMC article. Review. - Epithelial splicing regulatory protein 1 is a favorable prognostic factor in pancreatic cancer that attenuates pancreatic metastases.
Ueda J, Matsuda Y, Yamahatsu K, Uchida E, Naito Z, Korc M, Ishiwata T. Ueda J, et al. Oncogene. 2014 Sep 4;33(36):4485-95. doi: 10.1038/onc.2013.392. Epub 2013 Sep 30. Oncogene. 2014. PMID: 24077287 Free PMC article. - 3D Cancer Models: Depicting Cellular Crosstalk within the Tumour Microenvironment.
Franchi-Mendes T, Eduardo R, Domenici G, Brito C. Franchi-Mendes T, et al. Cancers (Basel). 2021 Sep 14;13(18):4610. doi: 10.3390/cancers13184610. Cancers (Basel). 2021. PMID: 34572836 Free PMC article. Review.
References
- Solcia E, Capella G, Klöppel G. Tumors of the exocrine pancreas. In: Rosai J, Sobin L, eds. Atlas of tumor pathology. Tumors of the pancreas. Washington DC: Armed Forces Institute of Pathology, 1997:64–88.
- Ryu B, Jones J, Hollingsworth MA, et al. Invasion-specific genes in malignancy: serial analysis of gene expression comparisons of primary and passaged cancers. Cancer Res 2001;61:1833–8. - PubMed
- Wong YC, Wang YZ. Growth factors and epithelial–stromal interactions in prostate cancer development. Int Rev Cytol 2000;199:65–116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous