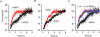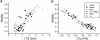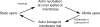AMPA-receptor activation regulates the diffusion of a membrane marker in parallel with dendritic spine motility in the mouse hippocampus - PubMed (original) (raw)
AMPA-receptor activation regulates the diffusion of a membrane marker in parallel with dendritic spine motility in the mouse hippocampus
D A Richards et al. J Physiol. 2004.
Abstract
Dendritic spines are the site of most excitatory connections in the hippocampus. We have investigated the diffusibility of a membrane-bound green fluorescent protein (mGFP) within the inner leaflet of the plasma membrane using Fluorescence Recovery After Photobleaching. In dendritic spines the diffusion of mGFP was significantly retarded relative to the dendritic shaft. In parallel, we have assessed the motility of dendritic spines, and found an inverse correlation between spine motility and the rate of diffusion of mGFP. We then tested the influence of glutamate receptor activation or blockade, and the involvement of the actin cytoskeleton (using latrunculin A) on spine motility and mGFP diffusion. These results show that glutamate receptors regulate the mobility of molecules in the inner leaflet of the plasma membrane through an action upon the actin cytoskeleton, suggesting a novel mechanism for the regulation of postsynaptic receptor density and composition.
Figures
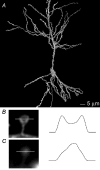
Figure 1. mGFP labels the surface of individual neurones in organotypic slices
A, 3D reconstruction of a living CA1 pyramidal neurone expressing mGFP. The cell was reconstructed from 60 optical sections taken at 0.5 μm intervals. Scale bar 5 μm. B, a maximum intensity projection of an individual spine labelled with mGFP. The image stack contained 16 sections at 0.25 μm intervals. The line indicates the position of a 1 μm fluorescence line profile, plotted to the right. Two maxima are seen, corresponding to the plasma membrane on either side of the spine. C, a maximum intensity projection of an individual spine labelled by iontophoretic injection of Lucifer yellow. Image obtained as in B. Note the bright fluorescence within the spine head, and the very dim spine neck. The line indicates the position of a 1 μm fluorescence line profile, plotted to the right.
Figure 2. Using FRAP to measure the mobility of mGFP in the inner membrane leaflet
A, time-lapse series showing the time course of FRAP following a 1 s bleach of the spot indicated by the white square. Time in seconds is indicated in the top right hand corner. B, time course of FRAP. Right hand panel, cartoon indicating the areas measured for comparison of FRAP of the spine head. Left hand panel, following bleach as indicated by the arrow, fluorescence recovered fully within 6–7 s in the spine head; fluorescence recovered faster in the spine neck, and bleach was barely detectable in the dendritic shaft.
Figure 3. Spine motility correlates with mGFP mobility in the membrane
A, spines were imaged over time and treated with glutamatergic agonist (AMPA) or antagonist (NBQX). AMPA-treated spines (red) showed faster recovery of fluorescence after bleaching than NBQX-treated spines (black). Filled circles represent means, thin lines indicate individual data sets. B, a series of experiments where spines were first imaged over time, classified as ‘static’ or ‘motile’, and then subjected to FRAP analysis. FRAP experiments were carried out at the end of imaging, and the recovery plotted against time. Static spines (red) show a faster recovery (smaller _t_½) than motile spines (black). C, treatment with latrunculin A (LA) speeds the time course of FRAP. In keeping with an effect downstream of the second messenger cascade (probably intracellular Ca2+) LA exerted this effect irrespective of the presence of NBQX (red indicates LA alone, blue indicates LA + NBQX). NBQX alone (black) is replotted from (A) for purposes of comparison.
Figure 4. Correlation between half-time of FRAP recovery and spine motility
A, scatter plot comparing spine motility with the _t_½ of FRAP. □, static spines, ▪, motile spines; •, AMPA treatment; ○, NBQX treatment; ▴, latrunculin treatment; ▾, spines treated with both latrunculin and NBQX. A strong correlation between spine motility and FRAP t_½ is seen. B, data from Fig. 4_A are plotted after further analysis. If each FRAP curve is analysed and fitted to a simple model of one-dimensional diffusion, the parameter D, diffusion coefficient can be determined. This is plotted against motility, giving a stronger correlation than simply plotting half-time of fluorescence recovery.
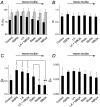
Figure 5. Comparison between simple and anomalous models of diffusion
A, the percentage recovery of fluorescence obtained by simple exponential fits (simple diffusion in black bars) and anomalous diffusion (grey bars) is compared. There is a modest correlation between motility and the values for R in the simple model, but this disappears in the anomalous diffusion model, where the additional component of diffusion provides a better fit of the data. B, the value for α, the anomalous diffusion coefficient, is largely unchanged under different conditions. C, the mean value for diffusion over rapid time scales (Df) shows a pronounced relation with motility. Statistical significance as assessd by t test is indicated by * where P < 0.05 and ** where P < 0.01. D, the mean value for diffusion over longer time scales (_D_s) shows a modest relation with motility. If anomalous diffusion itself was changing, then _D_s would be expected to change with no corresponding change in Df. Since this is not the case, and since α is also unchanged, we can conclude that the slowing of FRAP is due to a direct effect on the diffusion coefficient, presumeably due to the increased viscosity of the environment immediately adjacent to the membrane which results from actin polymerization.
Figure 6. Synaptic activity regulates spine motility through interactions between actin and the plasma membrane
A schematic diagram illustrates our model for the regulation of dendritic spine motility by glutamate. In the absence of synaptic activity (for example, during pharmacological blockade) a linkage is formed between the cytoskeleton and the inner leaflet of the plasma membrane; this retards diffusion in the lamina. Actin nucleation then drives changes in spine volume. Activation of glutamate receptors severs the linkage between actin and the membrane, speeding membrane diffusion and rendering spines static.
Comment in
- So, why do they dance, after all?
Segal M. Segal M. J Physiol. 2004 Jul 15;558(Pt 2):367. doi: 10.1113/jphysiol.2004.069260. Epub 2004 Jun 11. J Physiol. 2004. PMID: 15194732 Free PMC article. Review. No abstract available.
Similar articles
- Neuronal activity regulates diffusion across the neck of dendritic spines.
Bloodgood BL, Sabatini BL. Bloodgood BL, et al. Science. 2005 Nov 4;310(5749):866-9. doi: 10.1126/science.1114816. Science. 2005. PMID: 16272125 - Glutamate receptors regulate actin-based plasticity in dendritic spines.
Fischer M, Kaech S, Wagner U, Brinkhaus H, Matus A. Fischer M, et al. Nat Neurosci. 2000 Sep;3(9):887-94. doi: 10.1038/78791. Nat Neurosci. 2000. PMID: 10966619 - Activity of the AMPA receptor regulates drebrin stabilization in dendritic spine morphogenesis.
Takahashi H, Yamazaki H, Hanamura K, Sekino Y, Shirao T. Takahashi H, et al. J Cell Sci. 2009 Apr 15;122(Pt 8):1211-9. doi: 10.1242/jcs.043729. J Cell Sci. 2009. PMID: 19339553 - Signaling between the actin cytoskeleton and the postsynaptic density of dendritic spines.
Rao A, Craig AM. Rao A, et al. Hippocampus. 2000;10(5):527-41. doi: 10.1002/1098-1063(2000)10:5<527::AID-HIPO3>3.0.CO;2-B. Hippocampus. 2000. PMID: 11075823 Review. - Role of actin cytoskeleton in dendritic spine morphogenesis.
Sekino Y, Kojima N, Shirao T. Sekino Y, et al. Neurochem Int. 2007 Jul-Sep;51(2-4):92-104. doi: 10.1016/j.neuint.2007.04.029. Epub 2007 May 13. Neurochem Int. 2007. PMID: 17590478 Review.
Cited by
- Glutamate induces the rapid formation of spine head protrusions in hippocampal slice cultures.
Richards DA, Mateos JM, Hugel S, de Paola V, Caroni P, Gähwiler BH, McKinney RA. Richards DA, et al. Proc Natl Acad Sci U S A. 2005 Apr 26;102(17):6166-71. doi: 10.1073/pnas.0501881102. Epub 2005 Apr 14. Proc Natl Acad Sci U S A. 2005. PMID: 15831587 Free PMC article. - Barriers in the brain: resolving dendritic spine morphology and compartmentalization.
Adrian M, Kusters R, Wierenga CJ, Storm C, Hoogenraad CC, Kapitein LC. Adrian M, et al. Front Neuroanat. 2014 Dec 4;8:142. doi: 10.3389/fnana.2014.00142. eCollection 2014. Front Neuroanat. 2014. PMID: 25538570 Free PMC article. Review. - Glutamate receptor dynamics in dendritic microdomains.
Newpher TM, Ehlers MD. Newpher TM, et al. Neuron. 2008 May 22;58(4):472-97. doi: 10.1016/j.neuron.2008.04.030. Neuron. 2008. PMID: 18498731 Free PMC article. Review. - Dendritic spine viscoelasticity and soft-glassy nature: balancing dynamic remodeling with structural stability.
Smith BA, Roy H, De Koninck P, Grütter P, De Koninck Y. Smith BA, et al. Biophys J. 2007 Feb 15;92(4):1419-30. doi: 10.1529/biophysj.106.092361. Epub 2006 Nov 17. Biophys J. 2007. PMID: 17114228 Free PMC article. - Activity-dependent movements of postsynaptic scaffolds at inhibitory synapses.
Hanus C, Ehrensperger MV, Triller A. Hanus C, et al. J Neurosci. 2006 Apr 26;26(17):4586-95. doi: 10.1523/JNEUROSCI.5123-05.2006. J Neurosci. 2006. PMID: 16641238 Free PMC article.
References
- Akaaboune M, Grady RM, Turney S, Sanes JR, Lichtman JW. Neurotransmitter receptor dynamics studied in vivo by reversible photo-unbinding of fluorescent ligands. Neuron. 2002;34:865–876. - PubMed
- Caroni P. Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J Neurosci Meth. 1997;71:3–9. - PubMed
- Choquet D, Triller A. The role of receptor diffusion in the organization of the postsynaptic membrane. Nature Rev Neurosci. 2003;4:251–265. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources