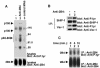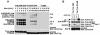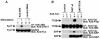Molecular dissection of 2B4 signaling: implications for signal transduction by SLAM-related receptors - PubMed (original) (raw)
Molecular dissection of 2B4 signaling: implications for signal transduction by SLAM-related receptors
Riyan Chen et al. Mol Cell Biol. 2004 Jun.
Abstract
2B4 is a SLAM-related receptor expressed on natural killer (NK) cells and cytotoxic T cells. It can regulate killing and gamma interferon secretion by NK cells, as well as T-cell-mediated cytotoxicity. There are conflicting data regarding the mechanism of action of 2B4. In these studies, we attempted to understand better the nature and basis of 2B4 signaling. Our studies showed that engagement of 2B4 on NK cells triggered a tyrosine phosphorylation signal implicating 2B4, Vav-1, and, to a lesser extent, SHIP-1 and c-Cbl. Structure-function analyses demonstrated that this response was defined by a series of tyrosine-based motifs in the cytoplasmic region of 2B4 and was not influenced by the extracellular or transmembrane segment of 2B4. In addition, the 2B4-induced signal was absolutely dependent on coexpression of SAP, a Src homology 2 (SH2) domain-containing adaptor associating with SLAM-related receptors and mutated in X-linked lymphoproliferative disease. It was also observed that 2B4 was detectably associated with the Src-related protein tyrosine kinase FynT in an immortalized NK cell line. Mutation of arginine 78 of SAP, a residue critical for binding of SAP to FynT, eliminated 2B4-mediated protein tyrosine phosphorylation, implying that SAP promotes 2B4 signaling most probably by recruiting FynT. Finally, despite the similarities in the signaling modalities of 2B4 and its relative SLAM, the natures of the tyrosine phosphorylation signals induced by these two receptors were found to be different. These differences were not caused by variations in the extent of binding to SAP but rather were dictated by the tyrosine-based sequences in the cytoplasmic domain of the receptors. Taken together, these data lead to a better understanding of 2B4 signaling. Furthermore, they provide firm evidence that the signals transduced by the various SLAM-related receptors are unique and that the specificity of these signals is defined by the distinctive arrays of intracytoplasmic tyrosines in the receptors.
Figures
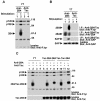
FIG. 1.
Engagement of 2B4 on YT cells induces intracellular protein tyrosine phosphorylation. (A) Overall intracellular protein tyrosine phosphorylation. YT cells were left unstimulated or stimulated for 10 min at 37°C with mouse anti-human 2B4 MAb C1.7 or an irrelevant antibody (mouse anti-Tac MAb 7G7), followed by RAM IgG. Unstimulated controls were incubated with RAM IgG alone. The accumulation of phosphotyrosine-containing proteins was determined by immunoblotting of total cell lysates with anti-P.tyr antibody. (B) Tyrosine phosphorylation of 2B4 and its association with SAP. This experiment was performed in parallel with that shown in panel A. After lysis in detergent-containing buffer, lysates were immunoprecipitated (I.P.) with the indicated antibodies and probed by immunoblotting with anti-P.tyr antibody (first part). The abundance of 2B4 in the immunoprecipitates was verified by reprobing of the immunoblot membrane with rabbit anti-human 2B4 serum (second part). The association of 2B4 with SAP was ascertained by probing of parallel 2B4 immunoprecipitates with polyclonal rabbit anti-human SAP antibody (third part). Lastly, the abundance of SAP was monitored by immunoblotting of total cell lysates with anti-SAP antibody (fourth part). (C) The cytoplasmic domain of 2B4 is sufficient to mediate intracellular protein tyrosine phosphorylation. Parental YT cells and YT derivatives expressing the indicated Tac-2B4 chimeras were left unstimulated or stimulated with anti-2B4 MAb C1.7 or anti-Tac MAb 7G7, as described for panel A. Changes in protein tyrosine phosphorylation were monitored by anti-P.tyr antibody immunoblotting (top). The abundance of the Tac-2B4 chimeras was verified by reprobing of the membrane with a polyclonal rabbit serum recognizing the extracellular segment of Tac (bottom). All cells expressed equivalent amounts of SAP (data not shown). The values to the right of panels A and C are molecular sizes in kilodaltons.
FIG. 2.
Identification of 2B4-regulated protein tyrosine phosphorylation substrates. YT cells expressing Tac-Tac-2B4 were left unstimulated or stimulated with biotinylated anti-Tac MAb 7G7 and avidin. After preclearing of cell lysates, potential substrates were immunoprecipitated with specific antibodies and tyrosine phosphorylation was assessed by immunoblotting with anti-P.tyr antibody. Reprobing of the immunoblot membrane with antibodies directed against the various substrates confirmed that all of the polypeptides were adequately immunoprecipitated under all of the conditions used (data not shown). The 70-kDa tyrosine-phosphorylated product seen in all lanes from anti-Tac antibody-stimulated cells is Tac-Tac-2B4, which was nonspecifically immunoprecipitated via the stimulating antibody. NRS, normal rabbit serum. The values to the right are molecular sizes in kilodaltons.
FIG. 3.
Analysis of 2B4-mediated protein tyrosine phosphorylation in IL-2-activated ex vivo mouse NK cells. (A) Overall protein tyrosine phosphorylation. Normal NK cells were isolated from mouse spleen and propagated in the presence of high concentrations of IL-2. After 7 to 10 days, cells were stimulated for 3 min with biotinylated MAb 2B4 (lane 2) or anti-TCR MAb F23.1 (lane 3), followed by avidin. Changes in protein tyrosine phosphorylation were determined by anti-P.tyr antibody immunoblotting (top). The abundance of 2B4 was assessed by reprobing with RAM 2B4 serum (bottom). (B) Tyrosine phosphorylation of SHIP-1 and Vav-1. The experiment was done as outlined for that shown in panel A, except that cells were left unstimulated or stimulated for 10 min. SHIP-1 (first and second parts) and Vav-1 (third and fourth parts) were immunoprecipitated (I.P.) from cell lysates with specific rabbit antisera. Their phosphotyrosine content was assessed by immunoblotting with anti-P.tyr antibody (first and third parts), whereas their abundance was verified by reprobing with anti-SHIP-1 antibody (second part) and anti-Vav-1 antibody (fourth part), respectively. (C) Tyrosine phosphorylation of 2B4. Mouse NK cells were left untriggered or triggered for the indicated periods of time with anti-2B4 MAb 2B4 and RAM IgG. After lysis, 2B4 was recovered by immunoprecipitation and probed by immunoblotting with anti-P.tyr (top) or anti-2B4 (bottom) antibody. The values to the right of panel A are molecular sizes in kilodaltons.
FIG. 4.
SAP expression is essential for 2B4-mediated protein tyrosine phosphorylation. Stable transfectants of BI-141 T cells expressing Tac-Tac-2B4, in the absence or presence of SAP, were stimulated for 10 min with anti-Tac MAb 7G7 and RAM IgG. (A) Tyrosine phosphorylation of Tac-Tac-2B4. The 2B4 chimera was immunoprecipitated (I.P.) from cell lysates and probed by immunoblotting with anti-P.tyr antibody (first part). The abundance of the chimera was verified by reprobing of the immunoblot membrane with rabbit anti-2B4 antibodies (second part), while the expression of SAP was confirmed by immunoblotting of total cell lysates with anti-SAP antibody (third part). (B) Overall protein tyrosine phosphorylation. Lysates from the experiment depicted in panel A were probed by anti-P.tyr antibody immunoblotting. The values to the right of panel B are molecular sizes in kilodaltons.
FIG. 5.
Analysis of 2B4-mediated signaling and function in XLP-derived NK cells. IL-2-activated NK cells derived from a normal individual or an asymptomatic XLP patient with a SAP R55L mutation were analyzed. (A) Flow cytometry. Expression of 2B4 and CD56 was assessed with anti-2B4 MAb C1.7 and an anti-CD56 MAb. APC, allophycocyanin; FITC, fluorescein isothiocyanate. (B) Cytotoxicity assay. The ability of NK cells to kill various targets was examined with a standard 51Cr release assay in the absence (−) or presence (+) of anti-2B4 antibody. The effector cell/target cell ratio used was 10:1. The extent of 51Cr release is shown on the abscissa. Maximum release (defined by lysis of target cells in detergent-containing buffer) is 100%. (C) 2B4-mediated overall protein tyrosine phosphorylation. Cells were stimulated for 10 min with anti-2B4 MAb C1.7 and SAM IgG. Changes in intracellular protein tyrosine phosphorylation were ascertained by immunoblotting of total cell lysates with anti-P.tyr antibody (top). The abundance of SAP was confirmed by immunoblotting of lysates with anti-SAP antibody (bottom). (D) Vav-1 tyrosine phosphorylation. The experiment was done as described for panel C, except that tyrosine phosphorylation of Vav-1 was determined by immunoblotting of total cell lysates with a phosphospecific antibody recognizing Vav-1 molecules phosphorylated at Y160 (top). The abundance of Vav-1 was verified by reprobing of the immunoblot membrane with anti-Vav-1 antibody (bottom). The values to the right of panel C are molecular sizes in kilodaltons.
FIG. 6.
Multiple tyrosines in the cytoplasmic domain of 2B4 are required for 2B4 signaling. (A) Schematic representation of Tac-Tac-2B4. The sequences of the four tyrosine-based motifs in mouse 2B4 with the consensus TxYxxV/I are indicated. (B) Impact of Y-to-F mutations on 2B4-mediated protein tyrosine phosphorylation. Parental YT cells or derivatives expressing the indicated Tac-Tac-2B4 variants were stimulated for 10 min with anti-Tac MAb 7G7 and RAM IgG. Changes in overall protein tyrosine phosphorylation were monitored by immunoblotting of total cell lysates with anti-P.tyr antibody (first part). The extent of tyrosine phosphorylation of the chimeras was also assessed by probing anti-Tac antibody immunoprecipitates (I.P.) with anti-P.tyr antibody (second part). The association of the chimeras with SAP was verified by immunoblotting of anti-Tac antibody immunoprecipitates with anti-SAP antibody (third part). The abundance of the Tac chimeras was confirmed by immunoblotting of anti-Tac antibody immunoprecipitates with anti-Tac antibody (fourth part), while the presence of SAP was assessed by immunoblotting of total cell lysates with anti-SAP antibody (fifth part). wt, wild type. The values to the right of panel B are molecular sizes in kilodaltons.
FIG. 7.
Different signals are transduced by the cytoplasmic domains of 2B4 and SLAM. (A) Overall protein tyrosine phosphorylation. BI-141 derivatives expressing the indicated Tac chimeras were stimulated for various times with biotinylated anti-Tac MAb 7G7 and avidin. Protein tyrosine phosphorylation was examined by immunoblotting of total cell lysates with anti-P.tyr antibody (top). The abundance of the chimeras was verified by reprobing of the immunoblot membrane with anti-Tac antibody (bottom). (B) Differential tyrosine phosphorylation of SHIP-1 and Vav-1. The experiment was as outlined for panel A, except that cells were stimulated for 5 min. Tyrosine phosphorylation of SHIP-1 was determined by probing of anti-SHIP-1 antibody immunoprecipitates (I.P.) with anti-P.tyr antibody (first part), whereas the abundance of SHIP-1 was verified by reprobing of the membrane with anti-SHIP-1 antibody (second part). The extent of tyrosine phosphorylation of Vav-1 was assessed by immunoblotting of total cell lysates with a phosphospecific antibody recognizing Vav-1 phosphorylated at Y160 (third part). The levels of Vav-1 were confirmed by reprobing of the membrane with anti-Vav-1 antibody (fourth part). Levels of SAP were compared by immunoblotting of total cell lysates with anti-SAP antibody (fifth part). The values to the right of panel A are molecular sizes in kilodaltons.
FIG. 8.
The specificity of SLAM-related receptor signaling is not defined by the extent of association with SAP. (A) Primary structure of Tac-Tac-SLAM-2B4. (B) Impact of the SAP-binding site from SLAM on 2B4 signaling. BI-141 cells expressing the indicated chimeras in the presence of SAP were stimulated for 10 min with anti-Tac MAb 7G7 as detailed in the legend to Fig. 7. The extent of intracellular protein tyrosine phosphorylation was measured by immunoblotting of total cell lysates with anti-P.tyr antibody (first part). The association of the chimeras with SAP was assessed by immunoblotting of anti-Tac antibody immunoprecipitates (I.P.) with anti-SAP antibodies (third part). The expression levels of the Tac chimeras and SAP were determined by immunoblotting of total cell lysates with anti-Tac (second part) and anti-SAP (fourth part) antibodies, respectively. (C) Expression of SLAM and SLAM-SLAM-2B4 on BI-141 cells. BI-141 cells expressing SAP were transduced with retroviruses encoding GFP alone (control) or in combination with full-length SLAM or a SLAM variant in which the cytoplasmic domain of SLAM was replaced with that of Tac-Tac-SLAM-2B4. GFP-positive cells were isolated by cell sorting and tested for cell surface expression of the two receptors with biotinylated anti-SLAM MAb 12F12 and streptavidin coupled to Quantum Red. Greater than 80% of the cells expressed SLAM or SLAM-SLAM-2B4. (D) Impact of SLAM and SLAM-SLAM-2B4 on TCR-induced IFN-γ production. Cells were stimulated overnight with the indicated concentrations of anti-CD3 MAb 145-2C11. The release of IFN-γ (in picograms per milliliter) in the supernatant was measured by enzyme-linked immunosorbent assay. Assays were done in duplicate, and the results shown are representative of at least five independent experiments. All cells expressed equivalent amounts of TCR complex at the cell surface (data not shown). The values to the right of panel B are molecular sizes in kilodaltons.
FIG. 9.
Evidence implicating the Src-related protein tyrosine kinase FynT in 2B4 signaling. (A) Association of 2B4 with activated FynT in YT cells. The presence of activated FynT was detected by reprobing the immunoblot membrane from Fig. 1B (first part) with an antibody that recognizes FynT molecules phosphorylated at Y417, the positive regulatory site (top). The expression levels of FynT were ascertained by immunoblotting of total cell lysates with anti-FynT antibody (bottom). (B) Arginine 78 of SAP is required for 2B4 tyrosine phosphorylation. BI-141 cells expressing Tac-Tac-2B4 with GFP alone (control) or in combination with wild-type (wt) or SAP R78A were stimulated with anti-Tac antibody in accordance with the protocol used for Fig. 7. Tyrosine phosphorylation of the chimera was determined by immunoblotting of anti-Tac antibody immunoprecipitates (I.P.) with anti-P.tyr antibody (first part). The presence of Tac-Tac-2B4 was confirmed by reprobing of the membrane with anti-2B4 antibody (second part). The association of Tac-Tac-2B4 with SAP was examined by probing anti-Tac antibody immunoprecipitates with anti-SAP antibody (third part), whereas the abundance of SAP was monitored by immunoblotting of total cell lysates with anti-SAP antibody (fourth part). Greater than 95% of the cells used for experimentation were GFP+ (data not shown).
FIG. 10.
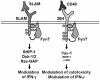
Model explaining the differential impact of 2B4 and SLAM on immune cell functions. A model for the different SAP-dependent impacts of two members of the SLAM family on immune cells is proposed. The ligand of SLAM is SLAM. In contrast, 2B4 interacts with CD48. See the text for further explanations.
Similar articles
- Potential pathways for regulation of NK and T cell responses: differential X-linked lymphoproliferative syndrome gene product SAP interactions with SLAM and 2B4.
Sayós J, Nguyen KB, Wu C, Stepp SE, Howie D, Schatzle JD, Kumar V, Biron CA, Terhorst C. Sayós J, et al. Int Immunol. 2000 Dec;12(12):1749-57. doi: 10.1093/intimm/12.12.1749. Int Immunol. 2000. PMID: 11099315 - Functional requirement for SAP in 2B4-mediated activation of human natural killer cells as revealed by the X-linked lymphoproliferative syndrome.
Tangye SG, Phillips JH, Lanier LL, Nichols KE. Tangye SG, et al. J Immunol. 2000 Sep 15;165(6):2932-6. doi: 10.4049/jimmunol.165.6.2932. J Immunol. 2000. PMID: 10975798 - Molecular basis for positive and negative signaling by the natural killer cell receptor 2B4 (CD244).
Eissmann P, Beauchamp L, Wooters J, Tilton JC, Long EO, Watzl C. Eissmann P, et al. Blood. 2005 Jun 15;105(12):4722-9. doi: 10.1182/blood-2004-09-3796. Epub 2005 Feb 15. Blood. 2005. PMID: 15713798 - 2B4: an NK cell activating receptor with unique specificity and signal transduction mechanism.
Nakajima H, Colonna M. Nakajima H, et al. Hum Immunol. 2000 Jan;61(1):39-43. doi: 10.1016/s0198-8859(99)00170-6. Hum Immunol. 2000. PMID: 10658976 Review. - NK cell regulation by SLAM family receptors and SAP-related adapters.
Veillette A. Veillette A. Immunol Rev. 2006 Dec;214:22-34. doi: 10.1111/j.1600-065X.2006.00453.x. Immunol Rev. 2006. PMID: 17100873 Review.
Cited by
- Activation, coactivation, and costimulation of resting human natural killer cells.
Bryceson YT, March ME, Ljunggren HG, Long EO. Bryceson YT, et al. Immunol Rev. 2006 Dec;214:73-91. doi: 10.1111/j.1600-065X.2006.00457.x. Immunol Rev. 2006. PMID: 17100877 Free PMC article. Review. - Structure of natural killer receptor 2B4 bound to CD48 reveals basis for heterophilic recognition in signaling lymphocyte activation molecule family.
Velikovsky CA, Deng L, Chlewicki LK, Fernández MM, Kumar V, Mariuzza RA. Velikovsky CA, et al. Immunity. 2007 Oct;27(4):572-84. doi: 10.1016/j.immuni.2007.08.019. Epub 2007 Oct 18. Immunity. 2007. PMID: 17950006 Free PMC article. - CD2 promotes human natural killer cell membrane nanotube formation.
Comerci CJ, Mace EM, Banerjee PP, Orange JS. Comerci CJ, et al. PLoS One. 2012;7(10):e47664. doi: 10.1371/journal.pone.0047664. Epub 2012 Oct 24. PLoS One. 2012. PMID: 23112830 Free PMC article. - Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion.
Bryceson YT, March ME, Ljunggren HG, Long EO. Bryceson YT, et al. Blood. 2006 Jan 1;107(1):159-66. doi: 10.1182/blood-2005-04-1351. Epub 2005 Sep 8. Blood. 2006. PMID: 16150947 Free PMC article. - Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells.
Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Bryceson YT, et al. J Exp Med. 2005 Oct 3;202(7):1001-12. doi: 10.1084/jem.20051143. J Exp Med. 2005. PMID: 16203869 Free PMC article.
References
- Aoukaty, A., and R. Tan. 2002. Association of the X-linked lymphoproliferative disease gene product SAP/SH2D1A with 2B4, a natural killer cell-activating molecule, is dependent on phosphoinositide 3-kinase. J. Biol. Chem. 277:13331-13337. - PubMed
- Benoit, L., X. Wang, H. F. Pabst, J. Dutz, and R. Tan. 2000. Defective NK cell activation in X-linked lymphoproliferative disease. J. Immunol. 165:3549-3553. - PubMed
- Bottino, C., R. Augugliaro, R. Castriconi, M. Nanni, R. Biassoni, L. Moretta, and A. Moretta. 2000. Analysis of the molecular mechanism involved in 2B4-mediated NK cell activation: evidence that human 2B4 is physically and functionally associated with the linker for activation of T cells. Eur. J. Immunol. 30:3718-3722. - PubMed
- Bottino, C., M. Falco, S. Parolini, E. Marcenaro, R. Augugliaro, S. Sivori, E. Landi, R. Biassoni, L. D. Notarangelo, L. Moretta, and A. Moretta. 2001. NTB-A, a novel SH2D1A-associated surface molecule contributing to the inability of natural killer cells to kill Epstein-Barr virus-infected B cells in X-linked lymphoproliferative disease. J. Exp. Med. 194:235-246. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous