The ubiquitin-conjugating DNA repair enzyme HR6A is a maternal factor essential for early embryonic development in mice - PubMed (original) (raw)
The ubiquitin-conjugating DNA repair enzyme HR6A is a maternal factor essential for early embryonic development in mice
Henk P Roest et al. Mol Cell Biol. 2004 Jun.
Abstract
The Saccharomyces cerevisiae RAD6 protein is required for a surprising diversity of cellular processes, including sporulation and replicational damage bypass of DNA lesions. In mammals, two RAD6-related genes, HR6A and HR6B, encode highly homologous proteins. Here, we describe the phenotype of cells and mice deficient for the mHR6A gene. Just like mHR6B knockout mouse embryonic fibroblasts, mHR6A-deficient cells appear to have normal DNA damage resistance properties, but mHR6A knockout male and female mice display a small decrease in body weight. The necessity for at least one functional mHR6A (X-chromosomal) or mHR6B (autosomal) allele in all somatic cell types is supported by the fact that neither animals lacking both proteins nor females with only one intact mHR6A allele are viable. In striking contrast to mHR6B knockout males, which show a severe spermatogenic defect, mHR6A knockout males are normally fertile. However, mHR6A knockout females fail to produce offspring despite a normal ovarian histology and ovulation. The absence of mHR6A in oocytes prevents development beyond the embryonic two-cell stage but does not result in an aberrant methylation pattern of histone H3 at this early stage of mouse embryonic development. These observations support redundant but dose-dependent roles for HR6A and HR6B in somatic cell types and germ line cells in mammals.
Figures
FIG. 1.
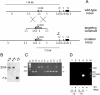
Targeted disruption of the mHR6A gene by homologous recombination. (A) Genomic organization and disruption strategy for mHR6A; shown are the gene, the targeting construct, and the targeted mHR6A allele. The neo cassette is inserted between the SalI site of exon 1 and the BamHI site in the first intron, introducing a diagnostic EcoRI site. Note that the insertion of the dominant marker disrupts the gene immediately behind the ATG translation initiation codon and deletes the coding part of exon 1. Shown are the relevant restriction sites (E, EcoRI; S, SalI; B, BamHI; C, ScaI; X, XmnI; H, HinDIII; O, XhoI; A, Asp718I; V, EcoRV). The position of the 3′ probe for Southern blot analysis is indicated above the mutated locus. Lines at the top and bottom indicate the estimated lengths of the fragments detected in Southern blot analysis of EcoRI-digested DNA. Roman numerals mark the exons. The 3′ deletion of exon 1 in the targeted allele is indicated with an asterisk. (B) Southern analysis of EcoRI-digested DNA from three neomycin-resistant ES clones after hybridization with the 3′ probe. The positions of the wild-type allele (23.6 kb) and the targeted allele (7.3 kb) are indicated. Note that the male ES line contains only one X-chromosomal mHR6A gene copy. “−/y” indicates a homologous recombinant ES clone, and “+/y” indicates a wild-type ES clone. The difference in signals between the targeted band and the wild-type band is presumably due to difficulty in the transfer of large fragments. (C) PCR-based genotyping of nine littermates. Primers mHR6A.7B and mHR6A.3B amplify a 152-bp fragment representing the wild-type allele (wt), and primers mHR6A.7B and NEOAS amplify a 473-nucleotide fragment representing the targeted allele (ta). Note that male animals (+/y and −/y) possess only one allele due to the X-chromosomal location of the allele. (D) RT PCR with total mouse testis RNA (lanes 1 to 4) from an _mHR6A_−/Y animal (lanes 1 and 2) and an mHR6A+/Y male littermate (lanes 3 and 4). Reactions were performed in the absence (lanes 1 and 3) or in the presence (lanes 2, 4, and 5) of RT. Lane 5 contains no RNA. M indicates the marker lane, with fragment lengths indicated on the right.
FIG. 2.
Paired t test of the difference between total body weights of mice with a specific genotype and control littermates 10 ± 1 days after birth. Each dot represents the average body weight of all animals with a specific genotype present in one litter. The mean body weights are indicated by horizontal bars. The asterisks indicate statistically significant differences between the indicated groups (P ≤ 0.05). N is the number of litters used in the analysis. (A) Total body weights of mHR6A-deficient animals and control littermates. Included are litters that contain both mHR6A+/Y and mHR6A_−/Y male mice and/or both mHR6A+/− and mHR6A_−/− female mice. (B) Total body weights of mice with one single functional mHR6B allele and control littermates. Included are only litters that contain both mHR6A+/Y/mHR6B+/+ and mHR6A_−/Y/mHR6B+/−_ male animals and/or both mHR6A+/− /mHR6B+/+ and mHR6A−/− /mHR6B+/− female animals. The genotypes of both genes are indicated under the x axis.
FIG. 3.
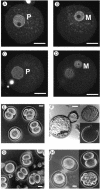
Developmental stages of preimplantation embryos obtained from mHR6A+/− (A, B, E, and F) and mHR6A−/− (C, D, G, and H) female mice fertilized by wild-type males. Images obtained with confocal laser scanning microscopy (A to D) show embryos from an mHR6A+/− heterozygous mother (A and B) and an mHR6A−/− knockout mother (C and D) approximately 8 h after fertilization. Two planes are displayed for the fertilized egg, one showing the paternal pronucleus (P in panels A and C) and one showing the maternal pronucleus (M in panels B and D). Phase-contrast microscopy shows examples of embryos obtained from mHR6A+/− (E and F) and mHR6A−/− (G and H) female mice at 1.5 dpc (E and G) and cultured for 3 days (F and H). (E) Embryos at various stages of development, obtained from an mHR6A+/− female. (F) Hatching (left) and hatched (upper right) embryos and an empty zona pellucida (lower right). (G) Embryos obtained from an mHR6A−/− female. (H) One-cell (left), two-cell (upper right), and fragmented (lower right) embryos. Scale bar, 20 μm.
FIG. 4.
mHR6A and mHR6B protein expression. Total protein extracts were separated by using two-dimensional gel electrophoresis. (A) Comparison of the amounts of mHR6A and mHR6B proteins (indicated by “A” and “B,” respectively) in ovaries from mHR6A+/+, mHR6A+/−, and mHR6A−/− mice. (B) Expression of mHR6A and mHR6B in oocytes isolated from wild-type mice compared to the amounts of the two proteins in whole ovaries.
FIG. 5.
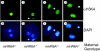
Immunofluorescent images of one-cell (A to D) and two-cell (E to H) embryos stained with an antibody against methylated H3K4 (A, C, E, and G) and DAPI (B, D, F, and H). The genotype of the mother is indicated on the right. The intense bright spots in panels C to H are caused by the remnants of a polar body. Staining of only one of the two pronuclei with α-methylated H3K4 is observed (A and C), while both nuclei of the two-cell embryo stain equally intensely (E and G).
Similar articles
- Histone H3-K9 methyltransferase ESET is essential for early development.
Dodge JE, Kang YK, Beppu H, Lei H, Li E. Dodge JE, et al. Mol Cell Biol. 2004 Mar;24(6):2478-86. doi: 10.1128/MCB.24.6.2478-2486.2004. Mol Cell Biol. 2004. PMID: 14993285 Free PMC article. - Histone ubiquitination and chromatin remodeling in mouse spermatogenesis.
Baarends WM, Hoogerbrugge JW, Roest HP, Ooms M, Vreeburg J, Hoeijmakers JH, Grootegoed JA. Baarends WM, et al. Dev Biol. 1999 Mar 15;207(2):322-33. doi: 10.1006/dbio.1998.9155. Dev Biol. 1999. PMID: 10068466 - Loss of HR6B ubiquitin-conjugating activity results in damaged synaptonemal complex structure and increased crossing-over frequency during the male meiotic prophase.
Baarends WM, Wassenaar E, Hoogerbrugge JW, van Cappellen G, Roest HP, Vreeburg J, Ooms M, Hoeijmakers JH, Grootegoed JA. Baarends WM, et al. Mol Cell Biol. 2003 Feb;23(4):1151-62. doi: 10.1128/MCB.23.4.1151-1162.2003. Mol Cell Biol. 2003. PMID: 12556476 Free PMC article. - The role of RAD6 in recombinational repair, checkpoints and meiosis via histone modification.
Game JC, Chernikova SB. Game JC, et al. DNA Repair (Amst). 2009 Apr 5;8(4):470-82. doi: 10.1016/j.dnarep.2009.01.007. Epub 2009 Feb 18. DNA Repair (Amst). 2009. PMID: 19230796 Review. - Oocyte-specific genes regulate follicle formation, fertility and early mouse development.
Dean J. Dean J. J Reprod Immunol. 2002 Jan;53(1-2):171-80. doi: 10.1016/s0165-0378(01)00100-0. J Reprod Immunol. 2002. PMID: 11730914 Review.
Cited by
- Zscan4: a novel gene expressed exclusively in late 2-cell embryos and embryonic stem cells.
Falco G, Lee SL, Stanghellini I, Bassey UC, Hamatani T, Ko MS. Falco G, et al. Dev Biol. 2007 Jul 15;307(2):539-50. doi: 10.1016/j.ydbio.2007.05.003. Epub 2007 May 8. Dev Biol. 2007. PMID: 17553482 Free PMC article. - Histone H2B monoubiquitination regulates heart development via epigenetic control of cilia motility.
Robson A, Makova SZ, Barish S, Zaidi S, Mehta S, Drozd J, Jin SC, Gelb BD, Seidman CE, Chung WK, Lifton RP, Khokha MK, Brueckner M. Robson A, et al. Proc Natl Acad Sci U S A. 2019 Jul 9;116(28):14049-14054. doi: 10.1073/pnas.1808341116. Epub 2019 Jun 24. Proc Natl Acad Sci U S A. 2019. PMID: 31235600 Free PMC article. - Transmission of Y chromosomes from XY female mice was made possible by the replacement of cytoplasm during oocyte maturation.
Obata Y, Villemure M, Kono T, Taketo T. Obata Y, et al. Proc Natl Acad Sci U S A. 2008 Sep 16;105(37):13918-23. doi: 10.1073/pnas.0802680105. Epub 2008 Sep 4. Proc Natl Acad Sci U S A. 2008. PMID: 18772381 Free PMC article. - Utility of DNA postreplication repair protein Rad6B in neoadjuvant chemotherapy response.
Shekhar MP, Biernat LA, Pernick N, Tait L, Abrams J, Visscher DW. Shekhar MP, et al. Med Oncol. 2010 Jun;27(2):466-73. doi: 10.1007/s12032-009-9235-7. Epub 2009 May 23. Med Oncol. 2010. PMID: 19466589 - Maternal effect genes: Findings and effects on mouse embryo development.
Kim KH, Lee KA. Kim KH, et al. Clin Exp Reprod Med. 2014 Jun;41(2):47-61. doi: 10.5653/cerm.2014.41.2.47. Epub 2014 Jun 30. Clin Exp Reprod Med. 2014. PMID: 25045628 Free PMC article. Review.
References
- Adenot, P. G., Y. Mercier, J. P. Renard, and E. M. Thompson. 1997. Differential H4 acetylation of paternal and maternal chromatin precedes DNA replication and differential transcriptional activity in pronuclei of 1-cell mouse embryos. Development 124:4615-4625. - PubMed
- Aoki, F., D. M. Worrad, and R. M. Schultz. 1997. Regulation of transcriptional activity during the first and second cell cycles in the preimplantation mouse embryo. Dev. Biol. 181:296-307. - PubMed
- Arney, K. L., S. Bao, A. J. Bannister, T. Kouzarides, and M. A. Surani. 2002. Histone methylation defines epigenetic asymmetry in the mouse zygote. Int. J. Dev. Biol. 46:317-320. - PubMed
- Auffray, C., and F. Rougeon. 1980. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur. J. Biochem. 107:303-314. - PubMed
- Baarends, W. M., J. W. Hoogerbrugge, H. P. Roest, M. Ooms, J. Vreeburg, J. H. Hoeijmakers, and J. A. Grootegoed. 1999. Histone ubiquitination and chromatin remodeling in mouse spermatogenesis. Dev. Biol. 207:322-333. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials