Akt mediates Ras downregulation of RhoB, a suppressor of transformation, invasion, and metastasis - PubMed (original) (raw)
Akt mediates Ras downregulation of RhoB, a suppressor of transformation, invasion, and metastasis
Kun Jiang et al. Mol Cell Biol. 2004 Jun.
Abstract
Although recent evidence supports a tumor-suppressive role for the GTPase RhoB, little is known about its regulation by signal transduction pathways. Here we demonstrate that Ras downregulates RhoB expression by a phosphatidylinositol 3-kinase (PI3K)- and Akt- but not Mek-dependent mechanism. Furthermore, genetic and pharmacological blockade of PI3K/Akt results in upregulation of RhoB expression. We also provide evidence for the importance of the downregulation of RhoB in oncogenesis by demonstrating that RhoB antagonizes Ras/PI3K/Akt malignancy. Ectopic expression of RhoB, but not the close relative RhoA, inhibits Ras, PI3K, and Akt induction of transformation, migration, and invasion and induces apoptosis and anoikis. Finally, RhoB inhibits melanoma metastasis to the lung in a mouse model. These studies identify suppression of RhoB as a mechanism by which the Ras/PI3K/Akt pathway induces tumor survival, transformation, invasion, and metastasis.
Figures
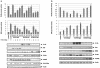
FIG. 1.
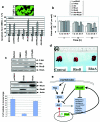
Oncogenic PI3K/Akt, but not Mek1/2 mediates Ras suppression of RhoB promoter transcriptional activity in NIH 3T3 cells. NIH 3T3 cells were transiently transfected with various oncogene constructs (H-Ras, DN-PI3K, DN-Akt1, DN-Mek1/2 in panel a and CA-PI3K, CA-Akt1, and CA-Mek1/2 in panel b) or pcDNA3 vector control along with full-length RhoB promoter-firefly luciferase reporter and SRE-Renilla luciferase reporter for 36 h, and the cell lysates were processed for determination of RhoB and SRE promoter activity (upper panels) or for determination of expression of transfected genes as well as their effects on Akt and ERK phosphorylation (lower panels) as described in Materials and Methods. The data are reported as ratios of luciferase to β-galactosidase from the cells transfected by the oncogene(s) over those in pcDNA3-transfected cells.
FIG. 2.
The ability of 5-FU to induce RhoB promoter activity is antagonized by oncogenic Ras, PI3K, and Akt in NIH 3T3 cells. (a) NIH 3T3 cells were transiently transfected with RhoB-promoter luciferase and SRE-luciferase constructs in the presence or absence of H-Ras61L, CA-PI3K, CA-Akt, or CA-MEK1/2 for 24 h, and then the cells were treated with either DMSO or 2.0 μM 5-FU for another 12 h. The cell lysates were processed as in Fig. 1. H-Ras/NIH 3T3 (b) and A549 cells (d) were transiently transfected with RhoB-promoter luciferase and SRE-luciferase constructs and cultured for 24 h; the cells were then treated with either DMSO, LY294002, 5-FU, or LY294002 plus 5-FU for 12 h. The cell lysates were processed as in Fig. 1. (c) PANC-1 cells were transiently transfected with CA-PI3K, CA-Akt, or pcDNA3 vector control for 24 h and then treated with either DMSO, LY294002, 5-FU, or LY294002 plus 5-FU for 12 h. The cell lysates were processed as in Fig. 1.
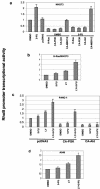
FIG. 3.
Blocking H-Ras/PI3K/Akt pathway induces RhoB expression in NIH 3T3 cells and human cancer cell lines. H-Ras**/**NIH 3T3 cells were treated with different concentrations of LY294002 for 48 h (a) or LY294002 for different time intervals (b). The cells were then lysed and analyzed for P-Akt, RhoB, and RhoA protein levels by Western blotting with anti-RhoB and anti-RhoA; the same filter was reprobed with anti-β-actin for a loading control. (c) NIH 3T3 cells were transiently transfected with various genes as indicated for 24 h and then treated with either DMSO vehicle, LY294002, or 5-FU alone or in combination for another 48 h. The cells were analyzed for P-Akt, RhoB, RhoA, and β-actin protein levels as described above. (d) PANC-1 cells were transiently transfected with DN-Akt1 and DN-Akt2 genes as indicated for 24 h and then treated with either DMSO vehicle or 5-FU alone or in combination for another 48 h. The cells were analyzed for P-Akt, RhoB, and RhoA protein levels by Western blotting with anti-P-Akt, anti-RhoB, and anti-RhoA; the same filter was reprobed with anti-β-actin, anti-HA, and anti-pan-Akt antibodies. PANC-1 (e) and A549 (f) cells were treated with PD, LY294002, or 5-FU as indicated for 48 h and then analyzed for RhoB and RhoA protein levels by Western blotting as described above.
FIG. 3.
Blocking H-Ras/PI3K/Akt pathway induces RhoB expression in NIH 3T3 cells and human cancer cell lines. H-Ras**/**NIH 3T3 cells were treated with different concentrations of LY294002 for 48 h (a) or LY294002 for different time intervals (b). The cells were then lysed and analyzed for P-Akt, RhoB, and RhoA protein levels by Western blotting with anti-RhoB and anti-RhoA; the same filter was reprobed with anti-β-actin for a loading control. (c) NIH 3T3 cells were transiently transfected with various genes as indicated for 24 h and then treated with either DMSO vehicle, LY294002, or 5-FU alone or in combination for another 48 h. The cells were analyzed for P-Akt, RhoB, RhoA, and β-actin protein levels as described above. (d) PANC-1 cells were transiently transfected with DN-Akt1 and DN-Akt2 genes as indicated for 24 h and then treated with either DMSO vehicle or 5-FU alone or in combination for another 48 h. The cells were analyzed for P-Akt, RhoB, and RhoA protein levels by Western blotting with anti-P-Akt, anti-RhoB, and anti-RhoA; the same filter was reprobed with anti-β-actin, anti-HA, and anti-pan-Akt antibodies. PANC-1 (e) and A549 (f) cells were treated with PD, LY294002, or 5-FU as indicated for 48 h and then analyzed for RhoB and RhoA protein levels by Western blotting as described above.
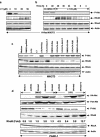
FIG. 4.
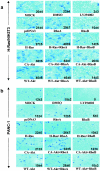
Ectopic expression of RhoB, not RhoA, inhibits Ras/PI3K/Akt-mediated transformation and migration. (a) Parental NIH 3T3 cells were transiently transfected with pcDNA3 vector control, H-Ras61L, CA-PI3K, or CA-Akt in the presence or absence of RhoA, RhoB, or pcDNA3 for 36 h. The cells were split, and an aliquot of the cells was evaluated for expression of transfected RhoA and RhoB by Western blotting. The rest of the cells were then cultured for another 4 weeks. The plates were examined for focus formation by staining. H-Ras/NIH 3T3 (b) and PANC-1 (c) cells were transiently transfected with pcDNA3 vector control, CA-Akt, or WT-Akt in the presence or absence of RhoA or RhoB or pcDNA3 for 36 h, internal ribosome entry site-EGFP was cotransfected as a transfection indicator. The cells were then split and analyzed for their migration capabilities through collagen type I-coated transfilters as described in Materials and Methods. (d and e) NIH 3T3 cells were transiently transfected with pcDNA3 vector control, H-Ras61L, CA-Akt, or WT-Akt in the presence or absence of RhoA, RhoB, or pcDNA3 for 36 h. The monolayers were then scratched with a yellow tip and microphotographed at the time points indicated to analyze their capability to migrate into and fill the wounded area.
FIG. 4.
Ectopic expression of RhoB, not RhoA, inhibits Ras/PI3K/Akt-mediated transformation and migration. (a) Parental NIH 3T3 cells were transiently transfected with pcDNA3 vector control, H-Ras61L, CA-PI3K, or CA-Akt in the presence or absence of RhoA, RhoB, or pcDNA3 for 36 h. The cells were split, and an aliquot of the cells was evaluated for expression of transfected RhoA and RhoB by Western blotting. The rest of the cells were then cultured for another 4 weeks. The plates were examined for focus formation by staining. H-Ras/NIH 3T3 (b) and PANC-1 (c) cells were transiently transfected with pcDNA3 vector control, CA-Akt, or WT-Akt in the presence or absence of RhoA or RhoB or pcDNA3 for 36 h, internal ribosome entry site-EGFP was cotransfected as a transfection indicator. The cells were then split and analyzed for their migration capabilities through collagen type I-coated transfilters as described in Materials and Methods. (d and e) NIH 3T3 cells were transiently transfected with pcDNA3 vector control, H-Ras61L, CA-Akt, or WT-Akt in the presence or absence of RhoA, RhoB, or pcDNA3 for 36 h. The monolayers were then scratched with a yellow tip and microphotographed at the time points indicated to analyze their capability to migrate into and fill the wounded area.
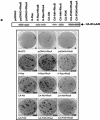
FIG. 5.
RhoB, but not RhoA, inhibits H-Ras/PI3K/Akt-mediated cell invasion. H-Ras/NIH 3T3 (a) and PANC-1 (b) cells were obtained from the samples shown in Fig. 4b and c that were transiently transfected with pcDNA3 vector control, H-Ras61L, CA-Akt, or WT-Akt in the presence or absence of RhoA or RhoB or pcDNA3 for 36 h. The cells were then split and analyzed for their invading capabilities through Matrigel-coated transfilters as described in Materials and Methods.
FIG. 6.
RhoB, but not RhoA, inhibits H-Ras/PI3K/Akt-mediated cell survival and metastasis. (a) Parental NIH 3T3 cells were transiently transfected with pcDNA3 vector, H-Ras61L, or Akt in the presence or absence of RhoA or RhoB or pcDNA3 for 36 h. Internal ribosome entry site-EGFP was cotransfected as a transfection indicator. The cells were then split and treated with 5-FU for another 48 h. The cells were thenexamined for their susceptibility to 5-FU-induced apoptosis by annexin V labeling and flow cytometry apoptosis assays as described in Materials and Methods. (b) Thirty-six hours after the transfection, a fraction of the cells from panel a were washed and resuspended in serum-free medium and then seeded onto poly-HEMA-precoated plates and examined for viability at different time points by annexin V and 7-AAD labeling. (c and d) B16-F10 melanoma cells were treated with LY294002 for 48 h, and 50 μg of the whole-cell lysates was analyzed for RhoB induction by Western blotting. In parallel, B16-F10 cells were transiently transfected with pcDNA3 vector, pcDNA-RhoA, or pcDNA-RhoB. Twenty hours later, the cells were examined for gene delivery by Western blotting with anti-HA, anti-RhoA, and anti-RhoB. A total of 5 × 105 of the cells were injected into the tail veins of C57/BL6 mice. The metastatic nodules growing in the lungs were counted and photographed at day 21. The numbers of metastatic nodules per mouse lung represent the average ± standard error from five mice per group.
Similar articles
- Geranylgeranylated, but not farnesylated, RhoB suppresses Ras transformation of NIH-3T3 cells.
Mazières J, Tillement V, Allal C, Clanet C, Bobin L, Chen Z, Sebti SM, Favre G, Pradines A. Mazières J, et al. Exp Cell Res. 2005 Apr 1;304(2):354-64. doi: 10.1016/j.yexcr.2004.10.019. Epub 2004 Dec 28. Exp Cell Res. 2005. PMID: 15748883 - EGFR, ErbB2 and Ras but not Src suppress RhoB expression while ectopic expression of RhoB antagonizes oncogene-mediated transformation.
Jiang K, Delarue FL, Sebti SM. Jiang K, et al. Oncogene. 2004 Feb 5;23(5):1136-45. doi: 10.1038/sj.onc.1207236. Oncogene. 2004. PMID: 14647415 - Autocrine signaling through Ras regulates cell survival activity in human glioma cells: potential cross-talk between Ras and the phosphatidylinositol 3-kinase-Akt pathway.
Sakata K, Kato S, Fox JC, Shigemori M, Morimatsu M. Sakata K, et al. J Neuropathol Exp Neurol. 2002 Nov;61(11):975-83. doi: 10.1093/jnen/61.11.975. J Neuropathol Exp Neurol. 2002. PMID: 12430714 - RhoB in cancer suppression.
Huang M, Prendergast GC. Huang M, et al. Histol Histopathol. 2006 Feb;21(2):213-8. doi: 10.14670/HH-21.213. Histol Histopathol. 2006. PMID: 16329046 Review. - Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy.
Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, Franklin RA, McCubrey JA. Chang F, et al. Leukemia. 2003 Mar;17(3):590-603. doi: 10.1038/sj.leu.2402824. Leukemia. 2003. PMID: 12646949 Review.
Cited by
- Proteome-wide Interrogation of Small GTPases Regulated by _N_6-Methyladenosine Modulators.
Yang YY, Yu K, Li L, Huang M, Wang Y. Yang YY, et al. Anal Chem. 2020 Jul 21;92(14):10145-10152. doi: 10.1021/acs.analchem.0c02203. Epub 2020 Jul 7. Anal Chem. 2020. PMID: 32567849 Free PMC article. - Arachidonic acid stimulates cell adhesion through a novel p38 MAPK-RhoA signaling pathway that involves heat shock protein 27.
Garcia MC, Ray DM, Lackford B, Rubino M, Olden K, Roberts JD. Garcia MC, et al. J Biol Chem. 2009 Jul 31;284(31):20936-45. doi: 10.1074/jbc.M109.020271. Epub 2009 Jun 8. J Biol Chem. 2009. PMID: 19506078 Free PMC article. - Resistin facilitates metastasis of lung adenocarcinoma through the TLR4/Src/EGFR/PI3K/NF-κB pathway.
Gong WJ, Liu JY, Yin JY, Cui JJ, Xiao D, Zhuo W, Luo C, Liu RJ, Li X, Zhang W, Zhou HH, Liu ZQ. Gong WJ, et al. Cancer Sci. 2018 Aug;109(8):2391-2400. doi: 10.1111/cas.13704. Epub 2018 Jul 20. Cancer Sci. 2018. PMID: 29927028 Free PMC article. - RasV12-mediated down-regulation of CCAAT/enhancer binding protein beta in immortalized fibroblasts requires loss of p19Arf and facilitates bypass of oncogene-induced senescence.
Sebastian T, Johnson PF. Sebastian T, et al. Cancer Res. 2009 Mar 15;69(6):2588-98. doi: 10.1158/0008-5472.CAN-08-2312. Epub 2009 Mar 10. Cancer Res. 2009. PMID: 19276382 Free PMC article. - The RhoB small GTPase in physiology and disease.
Vega FM, Ridley AJ. Vega FM, et al. Small GTPases. 2018 Sep 3;9(5):384-393. doi: 10.1080/21541248.2016.1253528. Epub 2016 Nov 22. Small GTPases. 2018. PMID: 27875099 Free PMC article. Review.
References
- Adnane, J., C. Muro-Cacho, L. Mathews, S. M. Sebti, and T. Munoz-Antonia. 2002. Suppression of rho B expression in invasive carcinoma from head and neck cancer patients. Clin. Cancer Res. 8:2225-2232. - PubMed
- Arboleda, M. J., J. F. Lyons, F. F. Kabbinavar, M. R. Bray, B. E. Snow, R. Ayala, M. Danino, B. Y. Karlan, and D. J. Slamon. 2003. Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer Res. 63:196-206. - PubMed
- Barbacid, M. 1987. ras genes. Annu. Rev. Biochem. 56:779-827. - PubMed
- Bouzahzah, B., C. Albanese, F. Ahmed, F. Pixley, M. P. Lisanti, J. D. Segall, J. Condeelis, D. Joyce, A. Minden, C. J. Der, A. Chan, M. Symons, and R. G. Pestell. 2001. Rho family GTPases regulate mammary epithelium cell growth and metastasis through distinguishable pathways. Mol. Med. 7:816-830. - PMC - PubMed
- Bowers, D. C., S. Fan, K. A. Walter, R. Abounader, J. A. Williams, E. M. Rosen, and J. Laterra. 2000. Scatter factor/hepatocyte growth factor protects against cytotoxic death in human glioblastoma via phosphatidylinositol 3-kinase- and AKT-dependent pathways. Cancer Res. 60:4277-4283. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources