6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: head-to-head with a bifunctional enzyme that controls glycolysis - PubMed (original) (raw)
Review
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: head-to-head with a bifunctional enzyme that controls glycolysis
Mark H Rider et al. Biochem J. 2004.
Abstract
Fru-2,6-P2 (fructose 2,6-bisphosphate) is a signal molecule that controls glycolysis. Since its discovery more than 20 years ago, inroads have been made towards the understanding of the structure-function relationships in PFK-2 (6-phosphofructo-2-kinase)/FBPase-2 (fructose-2,6-bisphosphatase), the homodimeric bifunctional enzyme that catalyses the synthesis and degradation of Fru-2,6-P2. The FBPase-2 domain of the enzyme subunit bears sequence, mechanistic and structural similarity to the histidine phosphatase family of enzymes. The PFK-2 domain was originally thought to resemble bacterial PFK-1 (6-phosphofructo-1-kinase), but this proved not to be correct. Molecular modelling of the PFK-2 domain revealed that, instead, it has the same fold as adenylate kinase. This was confirmed by X-ray crystallography. A PFK-2/FBPase-2 sequence in the genome of one prokaryote, the proteobacterium Desulfovibrio desulfuricans, could be the result of horizontal gene transfer from a eukaryote distantly related to all other organisms, possibly a protist. This, together with the presence of PFK-2/FBPase-2 genes in trypanosomatids (albeit with possibly only one of the domains active), indicates that fusion of genes initially coding for separate PFK-2 and FBPase-2 domains might have occurred early in evolution. In the enzyme homodimer, the PFK-2 domains come together in a head-to-head like fashion, whereas the FBPase-2 domains can function as monomers. There are four PFK-2/FBPase-2 isoenzymes in mammals, each coded by a different gene that expresses several isoforms of each isoenzyme. In these genes, regulatory sequences have been identified which account for their long-term control by hormones and tissue-specific transcription factors. One of these, HNF-6 (hepatocyte nuclear factor-6), was discovered in this way. As to short-term control, the liver isoenzyme is phosphorylated at the N-terminus, adjacent to the PFK-2 domain, by PKA (cAMP-dependent protein kinase), leading to PFK-2 inactivation and FBPase-2 activation. In contrast, the heart isoenzyme is phosphorylated at the C-terminus by several protein kinases in different signalling pathways, resulting in PFK-2 activation.
Figures
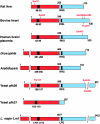
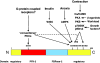
Figure 1. Domain organization of the subunit sequences of some of the PFK-2/FBPase-2 isoenzymes
The N-terminal PFK-2 domain is shown in red and the C-terminal FBPase-2 domain is in blue. Numbering refers to the individual isoenzyme sequences. The black box in the PFK-2 domain indicates the position of the glycine-rich loop in the nucleotide-binding fold with the signature G(X)4GKT/S. The black box in the FBPase-2 domain contains the active site histidine in the RHG motif which has become mutated in some of the monofunctional isoforms. Phosphorylation sites for protein kinases are indicated in red (see text).
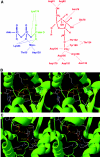
Figure 2. Residues involved in catalysis and substrate binding in the PFK-2 domain (A), stereo view of the location of critical residues for ATP-binding and catalysis (B) and Fru-6-P binding (C) in the 3D structure of the testis isoenzyme
The numbering of residues refers to the liver isoenzyme sequence. In (A), residues involved in substrate binding and catalysis in the PFK-2 domain are shown. In (B) and (C), the X-ray co-ordinates were retrieved from the PDB database (accession code 1BIF) corresponding to the testis isoenzyme containing ATPγS in the PFK-2 domain. The position of Fru-6-P was modelled as described [44]. The trace of the backbone is shown in green. Substrates and side-chains of critical residues are represented with carbon atoms in white, oxygen in red, nitrogen in blue and phosphate in yellow. Distances indicated by dotted lines are in Å.
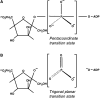
Figure 3. Associative and dissociative transition states for phosphotransferase catalysis in PFK-2
In the single displacement reaction, both substrates must be bound to the enzyme forming a ternary complex. Phosphoryl transfer can then take place either via (A) an associative transition state (SN2-like) or (B) a dissociative transition state (SN1-like).
Figure 4. 3D structure of the testis PFK-2/FBPase-2 homodimer (A) and close-up view of the head-to-head dimer interface (B)
(A) The co-ordinates were retrieved from the PDB database (accession code 1BIF). In this Figure, the PFK-2 domains are in green and come together in a head-to-head like fashion. The FBPase-2 domains are below in white. The α7 helices in each subunit are indicated by green arrows, and the α13 helices are in red (see text). The C-terminal ends in each subunit are indicated by yellow arrows. The side chains of residues in the PFK-2 domain shown in (B) are also shown in (A) and are coloured white in the upper domain. (B) Top view of the head-to-head interaction seen after turning the structure through 90 ° towards the reader along the plane of the β-sheet. Part of the interacting continuous 12-stranded β-sheet is shown together with the positions of some of the residues that differ between the testis and liver isoenzymes, namely Ser225 (replaced by arginine in the liver isoenzyme), Gln224 (replaced by threonine in the liver isoenzyme), along with Glu208, which is conserved (see text).
Figure 5. 3D structure of one enzyme subunit of the testis PFK-2/FBPase-2 isoenzyme
The co-ordinates were retrieved from the PDB database (accession code 1BIF) containing ATPγS in the PFK-2 domain. The position of Fru-6-P was modelled as described in [44]. In the upper right hand PFK-2 domain, ATP is on the left and Fru-6-P is on the right. In the lower left hand FBPase-2 domain, two inorganic phosphates indicate the position of the Fru-2,6-P2-binding site. The PFK-2 domain is composed of a β-sheet surrounded by α-helices. Two subdomains, composed of α-helices (above) form a flexible cover and are involved in Fru-6-P binding and catalysis (induced fit, see text).
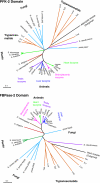
Figure 6. Phylogenetic trees constructed from sequences of the separate PFK-2 and FBPase-2 domains
Sequences of PFK-2 and/or FBPase-2s were aligned using the computer program ClustalX [208]. From this alignment, sub-alignments of either the PFK-2 domain or the FBPase-2 domain were made. The residues were from 42 to 248 for the PFK-2 domain and from 249 to 438 for the FBPase-2 domain (numbering refers to the rat liver isoenzyme). The sequences were yeast PFK-2 27 (no. Q12471), yeast PFK-2 26 (no. P40433), Neurospora crassa PFK-2/FBPase-2 (no. Q9P522), Schizosaccharomyces pombe PFK-2/FBPase-2 (nos. Q9UTE1, Q8TFH0), spinach (Spinacea oleracea) PFK-2/FBPase-2 (GenBank® accession no. AF041848), Arabidopsis thaliana PFK-2/FBPase-2 (GenBank® accession no. AF190739), potato (Solanum tuberosum) PFK-2/FBPase-2 (GenBank® accession no. AF073830), Leishmania major PFK-2 designated ‘Lm1’ (no. Q9NKN9), yeast FBPase-2 26 (no. P32604), Caenorhabditis elegans PFK-2/FBPase-2 (no. Q21122), Drosophila melanogaster PFK-2/FBPase-2 (no. Q9VWH7), human testis PFK-2/FBPase-2 (no. Q16877), rat testis PFK-2/FBPase-2 (no. P25114), bovine brain PFK-2/FBPase-2 (no. Q28901), human placenta PFK-2/FBPase-2 (no. Q16875), mouse placenta PFK-2/FBPase-2 (no. Q9ESY2), rat brain PFK-2/FBPase-2 (no. O35552), bovine heart PFK-2/FBPase-2 (no. P26285), human heart PFK-2/FBPase-2 (no. O60825), mouse heart PFK-2/FBPase-2 (no. P70265), rat heart PFK-2/FBPase-2 (no. Q9JJH5), chicken liver PFK-2/FBPase-2 (no. Q91348), seabream liver PFK-2/FBPase-2 (GenBank® accession no. U84724), bullfrog liver PFK-2/FBPase-2 (no. Q91309), rat liver PFK-2/FBPase-2 (no. P07953), bovine liver PFK-2/FBPase-2 (no. P49872), human liver PFK-2/FBPase-2 (no. P16118). The trypanosomatid sequences were retrieved from the relevant genome databases. The Desulphovibrio desulfuricans sequence (accession no. ZP_00131027) was taken from a BLAST search of the NCBI eubacterial genomes with the full-length rat liver PFK-2/FBPase-2 sequence. Accession numbers are for Swiss-Prot/TrEMBL unless stated otherwise. Phylogenetic trees were created using the tree option of the ClustalX program after exclusion of positions with gaps. Arabidopsis thaliana AK (no. Q9ZUU1) and E. coli phosphoglycerate mutase (no. P36942) were taken as outgroups to root the PFK-2 and FBPase-2 trees respectively. Horizontal bars represent ten substitutions per 100 residues.
Figure 7. Conserved C-terminal domains in the heart and brain/placenta PFK-2/FBPase-2 isoenzymes
The C-terminal sequence of human heart PFK-2/FBPase-2 containing the phosphorylation sites, Ser466 and Ser483, was aligned by eye with C-terminal sequences of the heart isoenzyme from other species and with the brain/placenta isoenzyme. The residue corresponding to Ser466 is in a suitable consensus for phosphorylation by AMPK in all sequences, whereas the brain/placenta isoenzyme lacks the −5 arginine residue for phosphorylation by insulin-stimulated protein kinases, although phosphorylation by PKA and PKC would still be expected (see text).
Figure 8. Potential protein kinase cascades converging on heart PFK-2/FBPase-2
The numbering of residues refers to the bovine heart isoenzyme. The signalling pathways leading to Ser466 phosphorylation of the heart isoenzyme could also impinge on Ser461 of the brain/placenta isoenzyme, but not on the equivalents of Ser84 or Thr475, which are replaced by non-phosphorylatable residues in the brain/placenta isoenzyme.
Similar articles
- 6-Phosphofructo-2-kinase and fructose-2,6-bisphosphatase in Trypanosomatidae. Molecular characterization, database searches, modelling studies and evolutionary analysis.
Chevalier N, Bertrand L, Rider MH, Opperdoes FR, Rigden DJ, Michels PA. Chevalier N, et al. FEBS J. 2005 Jul;272(14):3542-60. doi: 10.1111/j.1742-4658.2005.04774.x. FEBS J. 2005. PMID: 16008555 - A role for PFK-2/FBPase-2, as distinct from fructose 2,6-bisphosphate, in regulation of insulin secretion in pancreatic beta-cells.
Arden C, Hampson LJ, Huang GC, Shaw JA, Aldibbiat A, Holliman G, Manas D, Khan S, Lange AJ, Agius L. Arden C, et al. Biochem J. 2008 Apr 1;411(1):41-51. doi: 10.1042/BJ20070962. Biochem J. 2008. PMID: 18039179 - Cloning and expression in Escherichia coli of a rat hepatoma cell cDNA coding for 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase.
Crepin KM, Darville MI, Michel A, Hue L, Rousseau GG. Crepin KM, et al. Biochem J. 1989 Nov 15;264(1):151-60. doi: 10.1042/bj2640151. Biochem J. 1989. PMID: 2557826 Free PMC article. - Evolutionary analysis of fructose 2,6-bisphosphate metabolism.
Michels PA, Rigden DJ. Michels PA, et al. IUBMB Life. 2006 Mar;58(3):133-41. doi: 10.1080/15216540600688280. IUBMB Life. 2006. PMID: 16766380 Review. - Rat liver 6-phosphofructo 2-kinase/fructose 2,6-bisphosphatase: a review of relationships between the two activities of the enzyme.
El-Maghrabi MR, Pilkis SJ. El-Maghrabi MR, et al. J Cell Biochem. 1984;26(1):1-17. doi: 10.1002/jcb.240260102. J Cell Biochem. 1984. PMID: 6096384 Review.
Cited by
- Akt-dependent activation of the heart 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB2) isoenzyme by amino acids.
Novellasdemunt L, Tato I, Navarro-Sabate A, Ruiz-Meana M, Méndez-Lucas A, Perales JC, Garcia-Dorado D, Ventura F, Bartrons R, Rosa JL. Novellasdemunt L, et al. J Biol Chem. 2013 Apr 12;288(15):10640-51. doi: 10.1074/jbc.M113.455998. Epub 2013 Mar 2. J Biol Chem. 2013. PMID: 23457334 Free PMC article. - Multi-omics analysis to examine microbiota, host gene expression and metabolites in the intestine of black tiger shrimp (Penaeus monodon) with different growth performance.
Uengwetwanit T, Uawisetwathana U, Arayamethakorn S, Khudet J, Chaiyapechara S, Karoonuthaisiri N, Rungrassamee W. Uengwetwanit T, et al. PeerJ. 2020 Aug 14;8:e9646. doi: 10.7717/peerj.9646. eCollection 2020. PeerJ. 2020. PMID: 32864208 Free PMC article. - Cardiac Insulin Signaling Regulates Glycolysis Through Phosphofructokinase 2 Content and Activity.
Bockus LB, Matsuzaki S, Vadvalkar SS, Young ZT, Giorgione JR, Newhardt MF, Kinter M, Humphries KM. Bockus LB, et al. J Am Heart Assoc. 2017 Dec 4;6(12):e007159. doi: 10.1161/JAHA.117.007159. J Am Heart Assoc. 2017. PMID: 29203581 Free PMC article. - Neural Metabolic Networks: Key Elements of Healthy Brain Function.
Madrer N, Perera ND, Uccelli NA, Abbondanza A, Andersen JV, Carsana EV, Demmings MD, Fernandez RF, de Fragas MG, Gbadamosi I, Kulshrestha D, Lima-Filho RAS, Marian OC, Markussen KH, McGovern AJ, Neal ES, Sarkar S, Šimončičová E, Soto-Verdugo J, Yandiev S, Fernández-Moncada I. Madrer N, et al. J Neurochem. 2025 Jun;169(6):e70084. doi: 10.1111/jnc.70084. J Neurochem. 2025. PMID: 40454774 Free PMC article. Review. - Single-Cell RNA-Seq Analysis Links DNMT3B and PFKFB4 Transcriptional Profiles with Metastatic Traits in Hepatoblastoma.
Desterke C, Francés R, Monge C, Marchio A, Pineau P, Mata-Garrido J. Desterke C, et al. Biomolecules. 2024 Oct 31;14(11):1394. doi: 10.3390/biom14111394. Biomolecules. 2024. PMID: 39595571 Free PMC article.
References
- Van Schaftingen E. Fructose 2,6-bisphosphate. Adv. Enzymol. 1987;59:315–395. - PubMed
- Pilkis S. J., El-Maghrabi M. R., Claus T. H. Hormonal regulation of hepatic gluconeogenesis and glycolysis. Annu. Rev. Biochem. 1988;57:755–783. - PubMed
- Hue L., Rider M. H., Rousseau G. G. Fructose-2,6-bisphosphate in extra hepatic tissues. In: Pilkis S. J., editor. Fructose-2,6-bisphosphate. Boca Raton: CRC Press; 1990. pp. 173–193.
- Van Schaftingen E., Mertens E., Opperdoes F. R. Fructose-2,6-bisphosphate in primitive systems. In: Pilkis S. J., editor. Fructose-2,6-bisphosphate. Boca Raton: CRC Press; 1990. pp. 229–244.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials