Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms - PubMed (original) (raw)
Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms
Kuei Y Tseng et al. J Neurosci. 2004.
Abstract
Although the importance of dopamine (DA) for prefrontal cortical (PFC) cognitive functions is widely recognized, the nature of DA actions in the PFC remains controversial. A critical component in DA actions is its modulation of glutamate transmission, which can be different when specific receptors are activated. To obtain a clear picture of cellular mechanisms involved in these interactions, we studied the effects of DA-glutamate coactivation on pyramidal cell excitability in brain slices obtained from developmentally mature rats using whole-cell patch-clamp recordings. Bath application of NMDA, AMPA, and the D1 agonist SKF38393 induced concentration-dependent excitability increases, whereas bath application of the D2 receptor agonist quinpirole induced a concentration-dependent excitability decrease. The NMDA-mediated response was potentiated by SKF38393. This NMDA-D1 synergism required postsynaptic intracellular Ca2+ and protein kinase A (PKA) and was independent of membrane depolarization. On the other hand, the excitatory effects of both NMDA and AMPA were attenuated by a D2 agonist. Surprisingly, the D2-NMDA interaction was also blocked by the GABA(A) antagonists bicuculline and picrotoxin, suggesting that the inhibitory action of D2 receptors on NMDA-induced responses in the PFC may be mediated by GABAergic interneurons. In contrast, the D2-AMPA interaction involves inhibition of PKA and activation of phospholipase lipase C-IP3 and intracellular Ca2+ at a postsynaptic level. Thus, the modulatory actions of D1 and D2 receptors on PFC pyramidal cell excitability are mediated by multiple intracellular mechanisms and by activation of GABA(A) receptors, depending on the glutamate receptor subtypes involved.
Figures
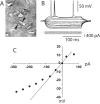
Figure 1.
Whole-cell recording from PFC pyramidal neurons. A, IR-DIC image of a layer V medial PFC pyramidal neuron recorded from a 300-μm-thick PFC slice (P52). The arrows point to the shadow of the recording electrode. B, Typical response to depolarizing and hyperpolarizing somatic current pulses (200 msec; from –300 to +100 pA). C, Current–voltage plot from the traces shown in B. Currents larger than –100 pA yielded a marked inward rectification.
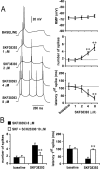
Figure 2.
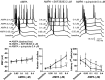
D1 activation enhances pyramidal cell excitability. A, Concentration-dependent excitability increases induced by bath application of SKF38393 in the PFC. Left panel, Representative tracings showing the increased action potential firing evoked by depolarizing current injection in the presence of 1, 2, 4, and 8 μ
m
SKF38393 concentrations. Right panel, Dose-dependent effect of SKF38393 on pyramidal cell excitability (measured as the latency to the first evoked spike and the number of spikes evoked by depolarizing current injection). The resting membrane potential was not significantly affected by bath application of SKF38393; however, the number of spikes was increased significantly and the latency to the first spike decreased with 4 and 8 μ
m
SKF38393 (compared with baseline; *p < 0.005, **p < 0.0002; repeated measures ANOVA).B, Bar graphs illustrating that the effects of 8 μ
m
SKF38393 on pyramidal cell excitability were blocked by the D1 antagonist SCH23390 (10 μ
m
). The excitatory action of 8 μ
m
SKF38393 on both the number of evoked spikes (left) and first spike latency were prevented by SCH23390 (compared with SKF38393 alone; **p < 0.005; repeated measures ANOVA).
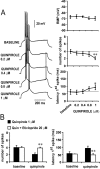
Figure 3.
D2 receptors decrease pyramidal cell excitability. A, Concentration-dependent excitability decreases induced by bath application of quinpirole in the PFC. Left panel, Traces recorded from a single neuron illustrating the effects of increasing quinpirole concentrations on action potential firing evoked by depolarizing current injection. Right panel, Dose-dependent effect of quinpirole on PFC pyramidal cell excitability. A significant decrease in the number of evoked spikes and increased first spike latency were observed only with 1 μ
m
quinpirole (compared with baseline; **p < 0.001, *p < 0.01; repeated measured ANOVA). No changes were observed in membrane potential. B, The inhibitory action of 1 μ
m
quinpirole on PFC pyramidal cell excitability was blocked by 20 μ
m
eticlopride (compared with quinpirole alone; **p < 0.001; repeated measures ANOVA).
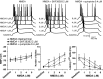
Figure 4.
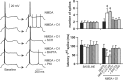
D1 and D2 modulation of NMDA effects on PFC cell excitability. Top, Overlay of tracings obtained with increasing NMDA concentrations (bottom to top) illustrating the effect of NMDA alone (left), NMDA plus SKF38393 (center), and NMDA plus quinpirole (right). Bottom, Graphs summarizing the effects of increasing NMDA concentrations on PFC pyramidal cell resting membrane potential (left), number of evoked spikes (center), and first spike latency (right). SKF38393 (2 μ
m
) significantly enhanced the effects of 1 and 2 μ
m
NMDA. In contrast, the effect of NMDA was significantly reduced in presence of quinpirole (0.4 μ
m
). *p < 0.03; **p < 0.002; Tukey post hoc test after significant ANOVA.
Figure 5.
D1 and D2 modulation of AMPA effects on PFC pyramidal cell excitability. Top, Overlays of traces obtained with increasing AMPA concentrations (bottom to top) illustrating the effects of AMPA alone (left), AMPA plus SKF38393 (center), and AMPA plus quinpirole (right). Bottom, Plots summarizing the effects of increasing AMPA concentrations (alone and in the presence of either a D1 or a D2 agonist) on PFC pyramidal cell resting membrane potential, number of evoked spikes, and latency to the first evoked spike. The excitatory effect of AMPA was attenuated in the presence of 0.4 μ
m
quinpirole. SKF38393 failed to change any AMPA-mediated electrophysiological action. *p < 0.03; **p < 0.001; Tukey post hoc test after significant ANOVA.
Figure 6.
The D1 enhancement of NMDA response is independent of membrane depolarization and requires intracellular Ca2+ and PKA activation. Left, Representative traces illustrating the response of a PFC pyramidal neuron to intracellular current injection after (from top to bottom) NMDA (1 μ
m
), NMDA plus SKF38393 (2 μ
m
), NMDA plus SKF38393 plus SCH23390 (10 μ
m
), NMDA plus SKF38393 plus the Ca2+ chelator BAPTA (2 m
m
), and NMDA plus SKF38393 plus the PKA blocker PKI-[5–24] (20 μ
m
). Right, Bar graphs summarizing the changes in number of evoked spikes and first spike latency observed in each experimental condition. The synergistic D1–NMDA effect on the number of evoked spikes and first spike latency was not affected by holding the resting membrane potential at baseline value (Vm adj). This potentiation was blocked by SCH23390 (10 μ
m
) and in recordings with electrodes containing BAPTA (2 m
m
) or PKI-[5–24] (20 μ
m
). *p < 0.001; Tukey post hoc test after significant ANOVA.
Figure 7.
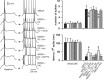
D2–NMDA interactions involve GABAA receptors. Left, Traces of responses to current injection illustrating changes in action potential firing observed in the different experimental conditions. From top to bottom, the traces are representative recordings obtained with NMDA alone (4 μ
m
), NMDA plus quinpirole (0.4 μ
m
), NMDA plus quinpirole plus the D2 antagonist eticlopride (20 μ
m
; eticlo), NMDA plus quinpirole plus the GABAA antagonist bicuculline (10 μ
m
; bic), NMDA plus quinpirole plus the GABAA antagonist picrotoxin (10 μ
m
; ptx), NMDA plus quinpirole plus BAPTA-containing electrode (2 m
m
), and NMDA plus PKI [5–24]-containing electrode (20 m
m
). Right, Bars graphs summarizing the changes in number of evoked spikes and first spike latency in each experimental condition. The inhibitory effect of quinpirole on NMDA responses was blocked by eticlopride (20 μ
m
; n = 5), by bicuculline (10 μ
m
; n = 6), and by picrotoxin (10 μ
m
; n = 6) but not in recordings with electrodes containing the calcium chelator BAPTA (n = 6). Intracellular application of the PKA inhibitor PKI-[5–24] (n = 5) failed to mimic the effects of quinpirole. *p < 0.001; Tukey post hoc test after significant ANOVA.
Figure 8.
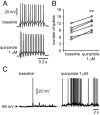
Quinpirole enhances interneuron excitability. A, Representative traces of responses to current injection showing the effect of quinpirole (1 μ
m
) on interneuron excitability. The number of evoked spikes increased from 12 to 15. B, Scatter graph summarizing the excitatory effect of quinpirole on PFC interneurons. The number of spikes evoked by intracellular current injection increased significantly in the presence of quinpirole 1 μ
m
(n = 6; **p < 0.001; paired t test). C, A pair of traces illustrating the membrane depolarization with increased firing rate induced by quinpirole.
Figure 9.
D2–AMPA interactions require intracellular Ca2+, PLC–IP3 pathway, and PKA inactivation. Left panel, Traces of responses to current injection showing the effects of AMPA on PFC pyramidal cell action potential firing obtained in different experimental conditions. The pairs of traces (baseline and treatment) are representative of (from top to bottom) experiments conducted with AMPA (0.2 μ
m
), AMPA plus quinpirole (0.4 μ
m
), AMPA plus quinpirole plus eticlopride (20 μ
m
; eticlo), AMPA plus quinpirole plus bicuculline (10 μ
m
; bic), AMPA plus quinpirole plus BAPTA (2 m
m
), AMPA plus PKI-[5–24] (20 μ
m
), AMPA plus quinpirole plus PKI-[5–24] (20 μ
m
), AMPA plus quinpirole plus the PLC blocker U73122 (10 μ
m
), and AMPA plus quinpirole plus the IP3 inhibitor Xec (2 μ
m
). Right, Bar graphs summarizing the changes in number of evoked spikes and first spike latency observed in each experimental condition. The effect of quinpirole on AMPA responses was blocked by eticlopride but not bicuculline. In the presence of PKI-[5–24], the excitatory effect of AMPA on PFC pyramidal cell excitability was partially attenuated. Recordings with electrodes containing BAPTA, U-73122, or Xestospongin-C were able to block the inhibitory action of D2 receptors on AMPA-mediated effects. Intracellular application of 0.15% DMSO did not alter the effects of quinpirole on AMPA responses (n = 5). *p < 0.001; Tukey post hoc test after significant ANOVA.
Similar articles
- Regulation of NMDA receptors by dopamine D4 signaling in prefrontal cortex.
Wang X, Zhong P, Gu Z, Yan Z. Wang X, et al. J Neurosci. 2003 Oct 29;23(30):9852-61. doi: 10.1523/JNEUROSCI.23-30-09852.2003. J Neurosci. 2003. PMID: 14586014 Free PMC article. - Post-pubertal disruption of medial prefrontal cortical dopamine-glutamate interactions in a developmental animal model of schizophrenia.
Tseng KY, Lewis BL, Lipska BK, O'Donnell P. Tseng KY, et al. Biol Psychiatry. 2007 Oct 1;62(7):730-8. doi: 10.1016/j.biopsych.2006.10.012. Epub 2007 Jan 3. Biol Psychiatry. 2007. PMID: 17207473 Free PMC article. - Dopamine D1 and D4 receptor subtypes differentially modulate recurrent excitatory synapses in prefrontal cortical pyramidal neurons.
Onn SP, Wang XB, Lin M, Grace AA. Onn SP, et al. Neuropsychopharmacology. 2006 Feb;31(2):318-38. doi: 10.1038/sj.npp.1300829. Neuropsychopharmacology. 2006. PMID: 16052247 - Dopamine/glutamate interaction as studied by combining turning behaviour and c-Fos expression.
Morelli M. Morelli M. Neurosci Biobehav Rev. 1997 Jul;21(4):505-9. doi: 10.1016/s0149-7634(96)00031-0. Neurosci Biobehav Rev. 1997. PMID: 9195609 Review. - Dopamine D1-like receptors and reward-related incentive learning.
Beninger RJ, Miller R. Beninger RJ, et al. Neurosci Biobehav Rev. 1998 Mar;22(2):335-45. doi: 10.1016/s0149-7634(97)00019-5. Neurosci Biobehav Rev. 1998. PMID: 9579323 Review.
Cited by
- The impact of adolescent social isolation on dopamine D2 and cannabinoid CB1 receptors in the adult rat prefrontal cortex.
Fitzgerald ML, Mackie K, Pickel VM. Fitzgerald ML, et al. Neuroscience. 2013 Apr 3;235:40-50. doi: 10.1016/j.neuroscience.2013.01.021. Epub 2013 Jan 16. Neuroscience. 2013. PMID: 23333674 Free PMC article. - Calcium-dependent networks in dopamine-glutamate interaction: the role of postsynaptic scaffolding proteins.
de Bartolomeis A, Tomasetti C. de Bartolomeis A, et al. Mol Neurobiol. 2012 Oct;46(2):275-96. doi: 10.1007/s12035-012-8293-6. Epub 2012 Jul 5. Mol Neurobiol. 2012. PMID: 22763587 Review. - Elevated prefrontal dopamine interferes with the stress-buffering properties of behavioral control in female rats.
McNulty CJ, Fallon IP, Amat J, Sanchez RJ, Leslie NR, Root DH, Maier SF, Baratta MV. McNulty CJ, et al. Neuropsychopharmacology. 2023 Feb;48(3):498-507. doi: 10.1038/s41386-022-01443-w. Epub 2022 Sep 8. Neuropsychopharmacology. 2023. PMID: 36076018 Free PMC article. - Dopamine receptor activation regulates reward expectancy signals during cognitive control in primate prefrontal neurons.
Ott T, Stein AM, Nieder A. Ott T, et al. Nat Commun. 2023 Nov 20;14(1):7537. doi: 10.1038/s41467-023-43271-6. Nat Commun. 2023. PMID: 37985776 Free PMC article. - Acute dopamine D(1) and D(2) receptor stimulation does not modulate mismatch negativity (MMN) in healthy human subjects.
Leung S, Croft RJ, Baldeweg T, Nathan PJ. Leung S, et al. Psychopharmacology (Berl). 2007 Nov;194(4):443-51. doi: 10.1007/s00213-007-0865-1. Epub 2007 Jul 5. Psychopharmacology (Berl). 2007. PMID: 17611739 Clinical Trial.
References
- Cepeda C, Levine MS (1998) Dopamine and N-methyl-d-aspartate receptor interactions in the neostriatum. Dev Neurosci 20: 1–18. - PubMed
- Cepeda C, Radisavljevic Z, Peacock W, Levine MS, Buchwald NA (1992) Differential modulation by dopamine of responses evoked by excitatory amino acids in human cortex. Synapse 11: 330–341. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous