A novel mechanism for the facilitation of theta-induced long-term potentiation by brain-derived neurotrophic factor - PubMed (original) (raw)
A novel mechanism for the facilitation of theta-induced long-term potentiation by brain-derived neurotrophic factor
Enikö A Kramár et al. J Neurosci. 2004.
Abstract
Brain-derived neurotrophic factor (BDNF) contributes to the induction of long-term potentiation (LTP) by theta-pattern stimulation, but the specific processes underlying this effect are not known. Experiments described here, using BDNF concentrations that have minor effects on baseline responses, show that the neurotrophin both reduces the threshold for LTP induction and elevates the ceiling on maximal potentiation. The enhanced LTP proved to be as stable and resistant to reversal as that recorded under control conditions. BDNF markedly increased the facilitation of burst responses that occurs within a theta train. This suggests that the neurotrophin acts on long-lasting events that (1) are set in motion by the first burst in a train and (2) regulate the amplitude of subsequent bursts. Whole-cell recordings established that BDNF causes a rapid reduction in the size of the long-lasting afterhyperpolarization (AHP) that follows individual theta bursts. Apamin, an antagonist of type 2 small-conductance Ca2+-activated potassium (SK2) channels, also reduced hippocampal AHPs and closely reproduced the effects of BDNF on theta-burst responses and LTP. The latter results were replicated with a newly introduced, highly selective inhibitor of SK2 channels. Immunoblot analyses indicated that BDNF increases SK2 serine phosphorylation in hippocampal slices. These findings point to the conclusion that BDNF-driven protein kinase cascades serve to depress the SK2 component, and possibly other constituents, of the AHP. It is likely that this mechanism, acting with other factors, promotes the formation and increases the magnitude of LTP.
Figures
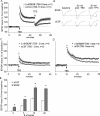
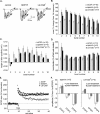
Figure 1.
Effects of BDNF on baseline synaptic transmission. A, BDNF at 2.0 n
m
was applied to the bath for 60 min (horizontal bar) after a stable 20 min baseline. The field EPSP slope for each slice was expressed as a percentage of the mean for the baseline period for that slice; shown is the mean ± SEM for a group of 11 slices. Field responses after 60 min of infusion showed no significant increase above baseline. Comparison of waveforms during baseline and at the 60 min time point indicated that BDNF caused a significant increase in the duration of the field EPSPs. Insets, Lowercase letters on the graph indicate when during the testing period the illustrated traces were collected. Calibration: 0.5 mV, 5 msec. B, Same as in A except that BDNF was infused along with 5 μ
m
PTX, an antagonist of GABAA receptors (n = 6). PTX did not amplify the effects of BDNF on the size or the waveform of the response. Lowercase letters indicate when during the recording period the traces were collected. Calibration: 0.4 mV, 5 msec.
Figure 2.
BDNF lowers the threshold and raises the ceiling of LTP induced by theta bursts. The slope of the field EPSP (mean ± SEM) was normalized to the mean of the last 60 responses collected in the presence of 2 n
m
BDNF before application of TBS (upward arrow). A, A train of 10 theta bursts produced a significantly greater degree of LTP in slices treated with BDNF than in control slices. At 30 min after LTP induction, three 1 min trains of 5 Hz stimulation (TPS, downward arrow), with the trains separated by 1 min, were delivered to the potentiated pathways. The theta-pulse stimulation caused a transient depression of LTP but this recovered over the subsequent 15 min. There were no evident differences in control and BDNF-treated slices with regard to recovery. B, Representative traces recorded from control and BDNF-treated slices during baseline, 30 min after TBS, and 30 min after TPS. Calibration: 1 mv, 5 msec. C, One pair of theta bursts was sufficient to induce LTP in BDNF-treated slices but not in control slices. D, A train of five theta bursts is twice as effective in inducing LTP in BDNF-treated slices as it is in controls. Note that the degree of potentiation in the former case is approximately equal to that generated by 10 bursts in control slices (A). E, Summary of grouped data (mean ± SEM) illustrating the effects of BDNF on the level of potentiation produced 30 min after applying 2, 5, 10, and 20 theta bursts. For each treatment, the BDNF group had significantly greater potentiation than the control slices (*p < 0.001).
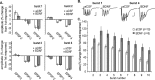
Figure 3.
Effects of BDNF on theta-burst responses. A, The percentage change in amplitude (relative to the first field EPSP) of the second, third, and fourth field EPSP within a single stimulus train of four pulses at 100 Hz in control and in 2 n
m
BDNF-treated slices (mean ± SEM). The measures are shown for bursts 1–4 of a train of 10 theta bursts. The overall shape of the response changes slightly across successive bursts; BDNF did not reliably affect burst topography. B, Traces collected after the delivery of the first and fourth theta bursts in control and BDNF-treated slices. Only the initial portions of the 200-msec-long responses are shown. Calibration: 1 mV, 10 msec. C, Percentage increase in response area across a series of 10 theta bursts in control and BDNF-treated slices. Within-train facilitation was significantly greater for each of the nine responses in the BDNF group (p < 0.01).
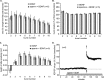
Figure 4.
Effects of BDNF on burst-response parameters. A, Representative examples of the first 50 msec of the first and fourth (double arrows) burst responses in a theta train. The negative-going component of the response lasts for ∼50 msec and is followed by a small positive potential (single arrow) that reaches its maximum at ∼200 msec (i.e., immediately before the next response in a train). BDNF increases both the size of the burst response and its duration. B, Mean ± SEM amplitudes of the afterpotential at 200 msec for groups of control and BDNF-treated slices; the amplitude for bursts 2–10 was significantly different in the two groups (p < 0.01). C, Comparison of the duration of the burst responses (measured as the time required for the response to return to the prestimulation baseline) in control and BDNF-treated slices. The mean ± SEM duration of responses across bursts 2–10 was significantly greater in the BDNF group (p < 0.01). D, Representative traces of the amplitude of the afterpositivity recorded during the second burst from a control and BDNF-treated slice. Traces depict the baseline and last 150 msec of the recorded response.
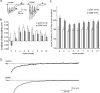
Figure 5.
Calcium-dependent AHPs are reduced by BDNF. A, Whole-cell recordings of theta-burst responses collected under control conditions and with electrodes containing the calcium-chelating agent BAPTA (20 m
m
). All recordings were made in the presence of PTX. Under control conditions, the theta burst triggers multiple spikes that are followed by an AHP (arrow) lasting 500–1000 msec. This afterpotential is greatly reduced in cells sampled with BAPTA electrodes. B, The AHP is reduced when the membrane potential is brought to –90 mV. C, Hyperpolarization is also triggered when brief injections of depolarizing current are used to elicit a short spike train (spikes are truncated in the figure). Bath infusions of apamin (50 n
m
), an inhibitor of SK2 channels, markedly reduce this potential, as indicated by the superimposed traces on the right (note that a higher gain was used in displaying these records; the AHP in the control trace is indicated by the arrow). D, Whole-cell recordings collected from control (top) and BDNF-treated (bottom) slices. The left trace was collected 5–10 min into the recording session, whereas the middle trace is from the 70 min time point. The two records are superimposed on the right. In all cases, the responses were initiated by a single theta burst. Local applications of BDNF (20 n
m
) via micropipette perfusion reduced the amplitude of the AHP (arrow indicates the afterpotential in the control trace). E, Mean ± SEM amplitude of the AHP for a group of four control slices in which aCSF was applied locally with a perfusion pipette beginning at 20 min and continued for the duration of the experiment. Theta bursts were applied once every 4 min. F, Effects of local applications of 20 n
m
BDNF beginning at 20 min for a group of 10 slices and continued for the duration of the experiment. The reduction in the mean ± SEM amplitude of the AHP beginning 15 min after the start of treatment was highly significant (p < 0.05). G, Summary of data for a subset of six slices in which the clamp was maintained for 70 min after the start of BDNF application; a late drop in the mean amplitude of the afterpotential is evident.
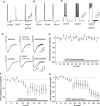
Figure 6.
SK2 channel blockers modify theta-burst responses. A, Representative examples of the first (single arrow) and the fourth (double arrows) responses to a train of theta bursts from three groups of slices: control; treated with the SK2 channel toxin apamin (50 n
m
); and treated with the specific SK2 channel antagonist Lei-Dab7 (100 n
m
). B, Apamin and Lei-Dab7 significantly increase the facilitation of burst responses that occur within a theta stimulation train relative to controls (p < 0.01). Shown are the mean ± SEM percentage increases in the areas of the responses to bursts 2–10 relative to the first burst in the train. C, Apamin and Lei-Dab7 both reduce the amplitude of the afterpositivity that follows the burst response; measurements were made at 200 msec after the beginning of the theta burst. D, The prolongation of burst responses that occur within a theta train is significantly greater in slices infused with apamin or Lei-Dab7 than it is in controls (p < 0.01). The values shown are mean ± SEM percentage changes from the duration of the first burst. E, LTP is enhanced in slices treated with 50 n
m
apamin or 100 n
m
Lei-Dab7. Graph shows representative experiments evaluating the effects of apamin and Lei-Dab7 on LTP. After a train of 10 theta bursts (TBS, upward arrow), the potentiation leveled off at ∼80%, 30 min after LTP induction. This effect is almost double the size of LTP in control slices.F, A single theta burst was applied to slices before and 60 min after treatment with 50 n
m
apamin or 100 n
m
Lei-Dab7. The percentage change in amplitude (relative to the first field EPSP) of the second, third, and fourth field EPSP within the single theta burst in control, 50 n
m
apamin-treated, and 100 n
m
Lei-Dab7-treated slices (mean ± SEM) is shown. The magnitude of the individual fEPSPs were no different within pretreatment and posttreatment conditions for both apamin- and Lei-Dab7-treated slices.
Figure 7.
Effects of a combination of apamin and BDNF on LTP and theta-burst responses. A, Slices treated with BDNF (2 n
m
) alone or BDNF plus apamin (50 n
m
) for 60 min before and after TBS had equivalent increases in the size of burst responses during a theta train. Note also that the pattern of increases (maximal for burst 3 and then decreasing over succeeding bursts) was the same for the two groups of slices. Areas for each burst were normalized to the area of the initial burst. B, Burst durations (percentage change from initial burst) were identical for the two groups of slices. C, The amplitudes of the slow, positive-going field potential that follows individual bursts were not different for slices treated with BDNF alone or BDNF plus apamin (p > 0.5; ANOVA). D, TBS (arrow) produced a robust LTP in slices treated with BDNF plus apamin. Potentiation at 50 min after TBS stabilized at +64 ± 5% above baseline. This value was not significantly different from that for slices treated with BDNF alone (p > 0.3).
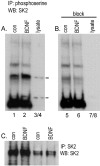
Figure 8.
BDNF treatment increases SK2 serine phosphorylation. A, B, Western blots of (1) phosphoserine immunoprecipitates from homogenates of control (con) and BDNF-treated hippocampal slices (lanes 1, 2, 5, 6) and (2) COS7 cell lysates (lanes 3/4, 7/8) that had been probed with intact anti-SK2 (Alomone Labs) (A) or anti-SK2 that had been preabsorbed with antigen peptide (B). As seen in A, two sharp bands of SK2 immunoreactivity within the COS7 lysate (lanes 3/4; at 105 and 45–50 kDa) correspond with phosphoprotein bands in the hippocampal homogenates that become markedly more robust with BDNF treatment. B shows that antigen-preabsorbed antibody no longer detects (1) these bands in the COS7 lysate or (2) enhanced phosphoserine immunoreactivity at these molecular weights in samples of BDNF-treated hippocampal tissue. C, Western blot of SK2 immunoprecipitates from homogenates of control and BDNF-treated slices that were probed with anti-SK2 (Alomone Labs) show that BDNF does not influence levels of total SK2 immunoreactivity; the two lanes at left and right were loaded with 30 and 10 μl of sample, respectively. IP, Immunoprecipitates; WB, whole brain.
Similar articles
- Increased small conductance calcium-activated potassium type 2 channel-mediated negative feedback on N-methyl-D-aspartate receptors impairs synaptic plasticity following context-dependent sensitization to morphine.
Fakira AK, Portugal GS, Carusillo B, Melyan Z, Morón JA. Fakira AK, et al. Biol Psychiatry. 2014 Jan 15;75(2):105-14. doi: 10.1016/j.biopsych.2013.04.026. Epub 2013 Jun 2. Biol Psychiatry. 2014. PMID: 23735878 Free PMC article. - Brain-derived neurotrophic factor triggers transcription-dependent, late phase long-term potentiation in vivo.
Messaoudi E, Ying SW, Kanhema T, Croll SD, Bramham CR. Messaoudi E, et al. J Neurosci. 2002 Sep 1;22(17):7453-61. doi: 10.1523/JNEUROSCI.22-17-07453.2002. J Neurosci. 2002. PMID: 12196567 Free PMC article. - Relative contribution of endogenous neurotrophins in hippocampal long-term potentiation.
Chen G, Kolbeck R, Barde YA, Bonhoeffer T, Kossel A. Chen G, et al. J Neurosci. 1999 Sep 15;19(18):7983-90. doi: 10.1523/JNEUROSCI.19-18-07983.1999. J Neurosci. 1999. PMID: 10479698 Free PMC article. - BDNF mechanisms in late LTP formation: A synthesis and breakdown.
Panja D, Bramham CR. Panja D, et al. Neuropharmacology. 2014 Jan;76 Pt C:664-76. doi: 10.1016/j.neuropharm.2013.06.024. Epub 2013 Jul 2. Neuropharmacology. 2014. PMID: 23831365 Review. - Theta-burst LTP.
Larson J, Munkácsy E. Larson J, et al. Brain Res. 2015 Sep 24;1621:38-50. doi: 10.1016/j.brainres.2014.10.034. Epub 2014 Oct 27. Brain Res. 2015. PMID: 25452022 Free PMC article. Review.
Cited by
- A tropomyosin-related kinase B ligand is required for ERK activation, long-term synaptic facilitation, and long-term memory in aplysia.
Sharma SK, Sherff CM, Stough S, Hsuan V, Carew TJ. Sharma SK, et al. Proc Natl Acad Sci U S A. 2006 Sep 19;103(38):14206-10. doi: 10.1073/pnas.0603412103. Epub 2006 Sep 8. Proc Natl Acad Sci U S A. 2006. PMID: 16963562 Free PMC article. - Temporal endurance of exercise-induced benefits on hippocampus-dependent memory and synaptic plasticity in female mice.
Dong TN, Kramár EA, Beardwood JH, Al-Shammari A, Wood MA, Keiser AA. Dong TN, et al. Neurobiol Learn Mem. 2022 Oct;194:107658. doi: 10.1016/j.nlm.2022.107658. Epub 2022 Jul 8. Neurobiol Learn Mem. 2022. PMID: 35811066 Free PMC article. - Regulation of hippocampal and behavioral excitability by cyclin-dependent kinase 5.
Hawasli AH, Koovakkattu D, Hayashi K, Anderson AE, Powell CM, Sinton CM, Bibb JA, Cooper DC. Hawasli AH, et al. PLoS One. 2009 Jun 4;4(6):e5808. doi: 10.1371/journal.pone.0005808. PLoS One. 2009. PMID: 19529798 Free PMC article. - Developmental profile of SK2 channel expression and function in CA1 neurons.
Ballesteros-Merino C, Lin M, Wu WW, Ferrandiz-Huertas C, Cabañero MJ, Watanabe M, Fukazawa Y, Shigemoto R, Maylie J, Adelman JP, Luján R. Ballesteros-Merino C, et al. Hippocampus. 2012 Jun;22(6):1467-80. doi: 10.1002/hipo.20986. Epub 2011 Nov 10. Hippocampus. 2012. PMID: 22072564 Free PMC article. - Enhanced ventral hippocampal synaptic transmission and impaired synaptic plasticity in a rodent model of alcohol addiction vulnerability.
Almonte AG, Ewin SE, Mauterer MI, Morgan JW, Carter ES, Weiner JL. Almonte AG, et al. Sci Rep. 2017 Sep 26;7(1):12300. doi: 10.1038/s41598-017-12531-z. Sci Rep. 2017. PMID: 28951619 Free PMC article.
References
- Ambros-Ingerson J, Granger R, Lynch G (1990) Simulation of paleocortex performs hierarchical clustering. Science 247: 1344–1348. - PubMed
- Aoki C, Wu K, Elste A, Len G, Lin S, McAuliffe G, Black IB (2000) Localization of brain-derived neurotrophic factor and TrkB receptors to postsynaptic densities of adult rat cerebral cortex. J Neurosci Res 59: 454–463. - PubMed
- Arai A, Lynch G (1992) Factors regulating the magnitude of long-term potentiation induced by theta pattern stimulation. Brain Res 598: 173–184. - PubMed
- Arai A, Black J, Lynch G (1994) Origins of the variations in long-term potentiation between synapses in the basal versus apical dendrites of hippocampal neurons. Hippocampus 4: 1–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous