Gene silencing of CENP-E by small interfering RNA in HeLa cells leads to missegregation of chromosomes after a mitotic delay - PubMed (original) (raw)
Gene silencing of CENP-E by small interfering RNA in HeLa cells leads to missegregation of chromosomes after a mitotic delay
Marcel Tanudji et al. Mol Biol Cell. 2004 Aug.
Abstract
Centromeric protein-E (CENP-E) is a kinesin-like motor protein required for chromosome congression at prometaphase. Functional perturbation of CENP-E by various methods results in a consistent phenotype, i.e., unaligned chromosomes during mitosis. One unresolved question from previous studies is whether cells complete mitosis or sustain mitotic arrest in the presence of unaligned chromosomes. Using RNA interference and video-microscopy, we analyzed the dynamic process of mitotic progression of HeLa(H2B)-GFP cells lacking CENP-E. Our results demonstrate that these cells initiated anaphase after a delayed mitotic progression due to the presence of unaligned chromosomes. In some dividing cells, unaligned chromosomes are present during anaphase, causing nondisjunction of some sister chromatids producing aneuploid daughter cells. Unlike in Xenopus extract, the loss of CENP-E in HeLa cells does not impair gross checkpoint activation because cells were arrested in mitosis in response to microtubule-interfering agents. However, the lack of CENP-E at kinetochores reduced the hyperphosphorylation of BubR1 checkpoint protein during mitosis, which may explain the loss of sensitivity of a cell to a few unaligned chromosomes in the absence of CENP-E. We also found that presynchronization with nocodazole sensitizes cells to the depletion of CENP-E, leading to more unaligned chromosomes, longer arrest, and cell death.
Figures
Figure 1.
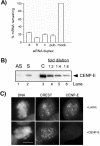
siRNA-induced reduction of CENP-E mRNA and protein levels. (A) Reduction of CENP-E mRNA. HeLa(H2B)-GFP cells were transfected with various CENP-E siRNA duplexes as indicated, and the mRNA levels were measured 24 h posttransfection by QPCR. pub., published siRNA duplex; mock, LipofectAMINE 2000 alone. (B) Analysis of CENP-E protein levels by Western blot. Seventy-five micrograms of mitotic extracts derived from asynchronous cells (lane 1, AS) or presynchronized cells (lane 2, S) 24 h after transfection with CENP-E siRNA was compared with twofold serial dilutions of control mitotic extracts starting at 75 μg (lanes 3–5). (C) Immunofluorescence of CENP-E of HeLa cells 24 h posttransfection with Lamin (control) or CENP-E siRNA. Cells were double stained with CREST and CENP-E antibodies. Identical exposure times were used for imaging both control and CENP-E siRNA-transfected cells. DNA was visualized with 4,6-diamidino-2-phenylindole staining. Bar, 10 μm.
Figure 2.
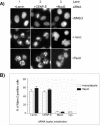
Effect of CENP-E depletion on mitotic progression. (A) Flow cytometry analysis of asynchronous HeLa(H2B)-GFP cells transfected with Lamin A/C (control) or CENP-E siRNA at 24, 48, and 72 h posttransfection. Cellular DNA content was determined by staining with propidium iodide. The percentage ranges of DNA contents at the three different time points are indicated on the upper right corner of each plot. (B) Live recordings of cells progressing through mitosis. HeLa(H2B)-GFP cells were transfected with Lamin (control) or CENP-E siRNA duplex, 24 h posttransfection. The morphological consequences were assessed by green fluorescent-chromosome/nucleus by using fluorescent time-lapse microscopy. Four different series of mitotic progression (i.e., normal [N], divide [D], died in mitosis [DM], and died after division [DD]) were observed and six images are shown for each series. Numbers in each panel denote the time (minutes) after the nuclear envelope breakdown (0 min). Asterisks denote the same daughter cells observed at different time points. Bar, 10 μm. (C) Anaphase occurs in the presence of unaligned chromosomes. HeLa(H2B)-GFP cells were transfected with CENP-E siRNA for 24 h and examined under a confocal fluorescent time-lapse microscope. The images were taken at 2-min intervals as indicated and show a cell with a pair of (top) or many unaligned chromosomes (bottom). Arrowheads denote unaligned chromosomes. Bar, 10 μm.
Figure 5.
Summary of the fate of mitotic cells with reduced levels of CENP-E. (A) Cell fate analysis of asynchronous HeLa(H2B)-GFP cells transfected with Lamin (control) or CENP-E siRNA duplex by using fluorescent videomicroscopy as described in Figure 2B. The number of cells without (N) or with unaligned chromosomes during mitosis were counted, and their fates were followed. (B) Same analysis as in A except that these cells were presynchronized with nocodazole before transfection. (C) Number of unaligned chromosomes in asynchronous and prenocodazole synchronized HeLa(H2B)-GFP cells 24 h after transfection with CENP-E siRNA duplex. 1
2 denotes more than two pairs unaligned sister chromatids. The data presented in A and B are the average of three independent experiments in each protocol. The data presented in C are the sum of all six experiments.
Figure 3.
CENP-E knockdown cells sustain the mitotic checkpoint in the presence of microtubule-interfering agents. (A) Images of green fluorescent-nuclei/chromosomes were taken after HeLa(H2B)-GFP cells were transfected with CENP-E, Lamin (negative control), and Mad2 siRNA (positive control) followed by either nocodazole or Taxol treatment for 16 h. Bar, 10 μm. (B) Flow cytometry profiles of HeLa cells with MPM-2 staining. HeLa cells were transfected with various siRNA duplexes and treated with nocodazole/Taxol as described in A. Subsequently, the cells were fixed, permeabilized, and stained with an antibody against the MPM-2 epitope, and the percentage of positive cells against fluorescent intensity was plotted. Cyc., untreated cycling cells.
Figure 4.
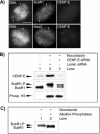
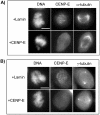
Effect of CENP-E reduction on checkpoint activation. (A) Immunofluorescence analysis for BubR1 (top) or Mad2 (bottom) and CENP-E in HeLa cells 24 h after transfection with CENP-E siRNA. Arrowheads denote unaligned chromosomes and fluorescent signal of BubR1 or Mad2 on these locations. Bar, 10 μm. (B) Phosphorylation of BubR1 is reduced in mitotic extracts of CENP-E siRNA-transfected cells. Middle, comparison of hyperphosphorylation status of hBubR1 in cycling extracts (lane 1), mitotic extracts of Lamin siRNA-transfected cells (lane 2), and mitotic extracts of CENP-E siRNA-transfected cells (lane 3). The samples also were examined for CENP-E (top) and phosphorylated histone H3 (bottom). (C) hBubR1 immunoprecipitated from mitotically blocked cell lysates was incubated without (lane 1) or with alkaline phosphatase (lane 2) (see MATERIALS AND METHODS). BubR1-P, hyperphosphorylated form; BubR1, unphosphorylated form.
Figure 6.
CENP-E knockdown does not affect spindle morphology. Images show immunofluorescence assay for CENP-E and α-tubulin (A) and CENP-E and γ-tubulin (B) in HeLa cells 24 h after transfection with Lamin or CENP-E siRNA. Bars, 10 μm.
Similar articles
- Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores.
Ditchfield C, Johnson VL, Tighe A, Ellston R, Haworth C, Johnson T, Mortlock A, Keen N, Taylor SS. Ditchfield C, et al. J Cell Biol. 2003 Apr 28;161(2):267-80. doi: 10.1083/jcb.200208091. J Cell Biol. 2003. PMID: 12719470 Free PMC article. - Septin 7 interacts with centromere-associated protein E and is required for its kinetochore localization.
Zhu M, Wang F, Yan F, Yao PY, Du J, Gao X, Wang X, Wu Q, Ward T, Li J, Kioko S, Hu R, Xie W, Ding X, Yao X. Zhu M, et al. J Biol Chem. 2008 Jul 4;283(27):18916-25. doi: 10.1074/jbc.M710591200. Epub 2008 May 6. J Biol Chem. 2008. PMID: 18460473 Free PMC article. - Human CENP-I specifies localization of CENP-F, MAD1 and MAD2 to kinetochores and is essential for mitosis.
Liu ST, Hittle JC, Jablonski SA, Campbell MS, Yoda K, Yen TJ. Liu ST, et al. Nat Cell Biol. 2003 Apr;5(4):341-5. doi: 10.1038/ncb953. Nat Cell Biol. 2003. PMID: 12640463 - Leaving no-one behind: how CENP-E facilitates chromosome alignment.
Craske B, Welburn JPI. Craske B, et al. Essays Biochem. 2020 Sep 4;64(2):313-324. doi: 10.1042/EBC20190073. Essays Biochem. 2020. PMID: 32347304 Free PMC article. Review. - Enhanced genomic instabilities caused by deregulated microtubule dynamics and chromosome segregation: a perspective from genetic studies in mice.
Rao CV, Yamada HY, Yao Y, Dai W. Rao CV, et al. Carcinogenesis. 2009 Sep;30(9):1469-74. doi: 10.1093/carcin/bgp081. Epub 2009 Apr 16. Carcinogenesis. 2009. PMID: 19372138 Free PMC article. Review.
Cited by
- CAML loss causes anaphase failure and chromosome missegregation.
Liu Y, Malureanu L, Jeganathan KB, Tran DD, Lindquist LD, van Deursen JM, Bram RJ. Liu Y, et al. Cell Cycle. 2009 Mar 15;8(6):940-9. doi: 10.4161/cc.8.6.7948. Epub 2009 Mar 26. Cell Cycle. 2009. PMID: 19229138 Free PMC article. - ShRNA-targeted centromere protein A inhibits hepatocellular carcinoma growth.
Li Y, Zhu Z, Zhang S, Yu D, Yu H, Liu L, Cao X, Wang L, Gao H, Zhu M. Li Y, et al. PLoS One. 2011 Mar 15;6(3):e17794. doi: 10.1371/journal.pone.0017794. PLoS One. 2011. PMID: 21423629 Free PMC article. - Micronuclei from misaligned chromosomes that satisfy the spindle assembly checkpoint in cancer cells.
Gomes AM, Orr B, Novais-Cruz M, De Sousa F, Macário-Monteiro J, Lemos C, Ferrás C, Maiato H. Gomes AM, et al. Curr Biol. 2022 Oct 10;32(19):4240-4254.e5. doi: 10.1016/j.cub.2022.08.026. Epub 2022 Sep 2. Curr Biol. 2022. PMID: 36057259 Free PMC article. - CTCF is essential for proper mitotic spindle structure and anaphase segregation.
Chiu K, Berrada Y, Eskndir N, Song D, Fong C, Naughton S, Chen T, Moy S, Gyurmey S, James L, Ezeiruaku C, Capistran C, Lowey D, Diwanji V, Peterson S, Parakh H, Burgess AR, Probert C, Zhu A, Anderson B, Levi N, Gerlitz G, Packard MC, Dorfman KA, Bahiru MS, Stephens AD. Chiu K, et al. Chromosoma. 2024 Jul;133(3):183-194. doi: 10.1007/s00412-023-00810-w. Epub 2023 Sep 20. Chromosoma. 2024. PMID: 37728741 - CTCF Recruits Centromeric Protein CENP-E to the Pericentromeric/Centromeric Regions of Chromosomes through Unusual CTCF-Binding Sites.
Xiao T, Wongtrakoongate P, Trainor C, Felsenfeld G. Xiao T, et al. Cell Rep. 2015 Sep 8;12(10):1704-14. doi: 10.1016/j.celrep.2015.08.005. Epub 2015 Aug 28. Cell Rep. 2015. PMID: 26321640 Free PMC article.
References
- Abrieu, A., Kahana, J.A., Wood, K.W., and Cleveland, D.W. (2000). CENP-E as an essential component of the mitotic checkpoint in vitro. Cell 102, 817-826. - PubMed
- Brown, K.D., Wood, K.W., and Cleveland, D.W. (1996). The kinesin-like protein CENP-E is kinetochore-associated throughout poleward chromosome segregation during anaphase-A. J. Cell Sci. 109, 961-969. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources