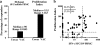Distinct time effects of vaccination on long-term proliferative and IFN-gamma-producing T cell memory to smallpox in humans - PubMed (original) (raw)
Distinct time effects of vaccination on long-term proliferative and IFN-gamma-producing T cell memory to smallpox in humans
Behazine Combadiere et al. J Exp Med. 2004.
Abstract
Residual immunity to the smallpox virus raises key questions about the persistence of long-term immune memory in the absence of antigen, since vaccination ended in 1980. IFN-gamma-producing effector-memory and proliferative memory T cells were compared in 79 vaccinees 13-25 yr after their last immunization and in unvaccinated individuals. Only 20% of the vaccinees displayed both immediate IFN-gamma-producing effector-memory responses and proliferative memory responses at 6 d; 52.5% showed only proliferative responses; and 27.5% had no detectable vaccinia-specific responses at all. Both responses were mediated by CD4 and CD8 T cells. The vaccinia-specific IFN-gamma-producing cells were composed mainly of CD4Pos CD45RANeg CD11aHi CD27Pos and CCR7Neg T cells. Their frequency was low but could be expanded in vitro within 7 d. Time since first immunization affected their persistence: they vanished 45 yr after priming, but proliferative responses remained detectable. The number of recalls did not affect the persistence of residual effector-memory T cells. Programmed revaccination boosted both IFN-gamma and proliferative responses within 2 mo of recall, even in vaccinees with previously undetectable residual effector-memory cells. Such long-term maintenance of vaccinia-specific immune memory in the absence of smallpox virus modifies our understanding of the mechanism of persistence of long-term memory to poxviruses and challenges vaccination strategies.
Figures
Figure 1.
Residual T cell–mediated immune responses to vaccinia virus in smallpox vaccinees. We examined fresh PBMCs from healthy individuals including 79 long-term vaccinees (VAC) (age, 25–68 yr; median, 39) and 10 unvaccinated unexposed volunteers (UNVAC) (age, 19–24 yr; median, 23). (a) Results from IFN-γ ELISpot assays after stimulation with the live Copenhagen vaccinia strain are represented as the percentage of healthy responders who are long-term vaccinees (black bars) (median for responders, 98; range, 53–843 SFCs/million PBMCs) and unvaccinated volunteers (white bar). Results from 3H-thymidine proliferation assays after 7 d stimulation with the live Copenhagen vaccinia strain are represented as the percentage of healthy responders who are long-term vaccinees (black bars) (median proliferation index, 15.5; range, 3–163); unvaccinated volunteers (white bars). (b) Reciprocal distributions of ELISpot and proliferation assays are represented for long-term vaccinees (•) and unvaccinated unexposed individuals (○). Positive responses were defined after analysis of responses obtained in unvaccinated control individuals: proliferative stimulation index ≥3 and IFN-γ–producing cells ≥50 SFCs/million PBMCs after subtraction of the background (shown by dashed lines). The percentage of vaccinees in each quadrant is indicated.
Figure 2.
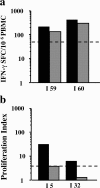
Vaccinia-specific CD4 and CD8 memory cells. CD8-depleted PBMCs (grey bars) compared with total PBMCs (black bars) were tested by ELISpot assay (a) and proliferation assay (b). Results are shown for two representative individuals.
Figure 3.
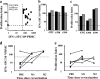
Vaccinia-specific CD4 and CD8 amplification leads to an increase in the frequency of effector–memory cells. (a) Percentage of BrdU+ cells in CD8 and CD4 T cells of five vaccinated individuals and two unvaccinated individuals after fresh PBMCs infected with either 1 pfu/cell vaccinia strain or medium alone were cultured for 3 d in the presence of BrdU (background subtracted). The number of IL-2–producing cells in the CD4 population was estimated by flow cytometric analysis at 48 h after antigenic stimulation (0–80 IL-2–producing cells/million CD4+ cells). (b) Representative flow cytometric analyses show IFN-γ production in vaccinia-specific T cells expanded for 7 d and restimulated on day 7 by monocytes infected with vaccinia for an additional 18 h at 1 pfu/cell (unvaccinated and long-term vaccinated individuals).
Figure 4.
Phenotypic analyses of vaccinia-specific memory T cells. Intracytoplasmic IFN-γ production by flow-cytometry assays of CD4 T cells after 18 h stimulation with vaccinia virus in selected volunteers with high frequencies of vaccinia-specific cells (>200 SFC/million PBMCs) (a–c, left panels top quadrant, unstimulated cells; bottom quadrant, vaccinia-stimulated cells). (a) CD45RA and CD11a expression gated on total CD4Pos cells or CD4PosIFN-γPos cells (in two individuals, I5 and I12; 78 and 70% of vaccinia-specific IFN-γ–producing CD4 cells were CD45RANegCD11aPos). N, naive T cells; E, effector T cells; M, memory T cells. Flow cytometry analyses for CCR7 expression showed that N, CCR7Pos, E, CCR7Neg, and M, CCR7Pos/Neg (not depicted). (b) CD45RA and CD27 expression are gated on either total CD4Pos cells or CD4PosIFN-γPos cells. Experiments were performed with five individuals: I1, I2, I5, I7, and I9 (60–70% of vaccinia-specific CD4 cells were CD45RANegCD27Pos). (c) CD45RA and CCR7 expression are gated on either total CD4Pos cells or CD4PosIFN-γPos cells. Experiments were performed with two individuals: I1 and I2 (80–91% of vaccinia-specific CD4 cells were CD45RANegCCR7Neg).
Figure 5.
Vaccinia-specific effector–memory response vanishes 45 yr after priming. (a and b) Distribution of vaccinia-specific responses in vaccinated individuals according to time since priming for ELISpot assay (a) and proliferation assay (b). Three groups were distinguished according to time since priming, 25–35, 36–45, and >45 yr, and were compared with unvaccinated unexposed donors. A precise, documented vaccination history was available for only 44 individuals as follows: one (n = 12), two (n = 23), three (n = 4), four (n = 4), or five (n = 1) immunizations. For all graphs, statistical analysis used the χ2 test. Statistical significance was set at P < 0.05 (**). ns, not significant.
Figure 6.
Lack of influence of vaccinia recalls and time since last immunization on the long-term persistence of IFN-γ–producing effector–memory T cells. Distribution of vaccinia-specific responses in 44 vaccinated individuals with a known vaccination history, according to the number of recalls they received up to 13 yr ago for both ELISpot (a) and proliferation (b) assays and according to time since last immunization for ELISpot (c) and proliferation (d) assays. The same three groups described in Fig. 3 were distinguished according to time since priming: 10–25, 25–45, and >45 yr. For all graphs, statistical analysis was performed as in Fig. 5.
Figure 7.
Recent vaccinia recall allows a complete immune response recovery in vaccinees independently of the time since priming. (a) Reciprocal distributions of ELISpot and proliferation assays for 17 recently revaccinated individuals (age, 32–57 yr; median, 41) tested 2 mo after revaccination. Positive responses were defined according to the same criteria: a stimulation index ≥3 and a frequency of IFN-γ–producing cells ≥50 SFC/million PBMCs after subtraction of background (shown by dashed lines). (b and c) CD8-depleted PBMCs of recently revaccinated individuals (grey bars) compared with total PBMCs (black bars) were tested by ELISpot (b) and proliferation (c) assays. Graphs represent changes in IFN-γ production (d) and proliferative responses (e) of five representative recently revaccinated individuals: PRE (before revaccination, 1–2 mo) M1 and M2 (after revaccination).
Similar articles
- Induction of human T cell-mediated immune responses after primary and secondary smallpox vaccination.
Kennedy JS, Frey SE, Yan L, Rothman AL, Cruz J, Newman FK, Orphin L, Belshe RB, Ennis FA. Kennedy JS, et al. J Infect Dis. 2004 Oct 1;190(7):1286-94. doi: 10.1086/423848. Epub 2004 Aug 30. J Infect Dis. 2004. PMID: 15346340 Clinical Trial. - Primary induction of human CD8+ cytotoxic T lymphocytes and interferon-gamma-producing T cells after smallpox vaccination.
Ennis FA, Cruz J, Demkowicz WE Jr, Rothman AL, McClain DJ. Ennis FA, et al. J Infect Dis. 2002 Jun 1;185(11):1657-9. doi: 10.1086/340517. Epub 2002 May 17. J Infect Dis. 2002. PMID: 12023773 Clinical Trial. - The persistence of humoral and cellular immunities more than three decades after smallpox vaccination.
Kim SH, Yeo SG, Park KH, Bang JW, Kim HB, Kim NJ, Jee Y, Cho H, Oh MD, Choe KW. Kim SH, et al. Clin Microbiol Infect. 2007 Jan;13(1):91-3. doi: 10.1111/j.1469-0691.2006.01576.x. Clin Microbiol Infect. 2007. PMID: 17184294 - Immunity and immunological memory following smallpox vaccination.
Amanna IJ, Slifka MK, Crotty S. Amanna IJ, et al. Immunol Rev. 2006 Jun;211:320-37. doi: 10.1111/j.0105-2896.2006.00392.x. Immunol Rev. 2006. PMID: 16824139 Review. - Old and new: recent innovations in vaccine biology and skin T cells.
Kupper TS. Kupper TS. J Invest Dermatol. 2012 Mar;132(3 Pt 2):829-34. doi: 10.1038/jid.2011.400. Epub 2012 Jan 12. J Invest Dermatol. 2012. PMID: 22237702 Free PMC article. Review.
Cited by
- Genome-wide analysis of polymorphisms associated with cytokine responses in smallpox vaccine recipients.
Kennedy RB, Ovsyannikova IG, Pankratz VS, Haralambieva IH, Vierkant RA, Poland GA. Kennedy RB, et al. Hum Genet. 2012 Sep;131(9):1403-21. doi: 10.1007/s00439-012-1174-2. Epub 2012 May 19. Hum Genet. 2012. PMID: 22610502 Free PMC article. - Human CD4+ T cell epitopes from vaccinia virus induced by vaccination or infection.
Calvo-Calle JM, Strug I, Nastke MD, Baker SP, Stern LJ. Calvo-Calle JM, et al. PLoS Pathog. 2007 Oct 12;3(10):1511-29. doi: 10.1371/journal.ppat.0030144. PLoS Pathog. 2007. PMID: 17937498 Free PMC article. - Control of vaccinia virus skin lesions by long-term-maintained IFN-gamma+TNF-alpha+ effector/memory CD4+ lymphocytes in humans.
Puissant-Lubrano B, Bossi P, Gay F, Crance JM, Bonduelle O, Garin D, Bricaire F, Autran B, Combadière B. Puissant-Lubrano B, et al. J Clin Invest. 2010 May;120(5):1636-44. doi: 10.1172/JCI38506. Epub 2010 Apr 1. J Clin Invest. 2010. PMID: 20364089 Free PMC article. - Rapamycin enhances BCG-specific γδ T cells during intravesical BCG therapy for non-muscle invasive bladder cancer: a randomized, double-blind study.
Ji N, Mukherjee N, Reyes RM, Gelfond J, Javors M, Meeks JJ, McConkey DJ, Shu ZJ, Ramamurthy C, Dennett R, Curiel TJ, Svatek RS. Ji N, et al. J Immunother Cancer. 2021 Mar;9(3):e001941. doi: 10.1136/jitc-2020-001941. J Immunother Cancer. 2021. PMID: 33653802 Free PMC article. Clinical Trial. - Genome-wide genetic associations with IFNγ response to smallpox vaccine.
Kennedy RB, Ovsyannikova IG, Pankratz VS, Haralambieva IH, Vierkant RA, Jacobson RM, Poland GA. Kennedy RB, et al. Hum Genet. 2012 Sep;131(9):1433-51. doi: 10.1007/s00439-012-1179-x. Epub 2012 Jun 3. Hum Genet. 2012. PMID: 22661280 Free PMC article.
References
- Henderson, D.A. 1999. Lessons from the eradication campaigns. Vaccine. 17:S53–S55. - PubMed
- Ahmed, R. 1996. Tickling memory T cells. Science. 272:1904. - PubMed
- Kaech, S.M., E.J. Wherry, and R. Ahmed. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2:251–262. - PubMed
- Sprent, J., and C.D. Surh. 2001. Generation and maintenance of memory T cells. Curr. Opin. Immunol. 13:248–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials