Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome - PubMed (original) (raw)
Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome
Robert D Goldman et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Hutchinson-Gilford progeria syndrome (HGPS) is a premature aging disorder, commonly caused by a point mutation in the lamin A gene that results in a protein lacking 50 aa near the C terminus, denoted LADelta50. Here we show by light and electron microscopy that HGPS is associated with significant changes in nuclear shape, including lobulation of the nuclear envelope, thickening of the nuclear lamina, loss of peripheral heterochromatin, and clustering of nuclear pores. These structural defects worsen as HGPS cells age in culture, and their severity correlates with an apparent increase in LADelta50. Introduction of LADelta50 into normal cells by transfection or protein injection induces the same changes. We hypothesize that these alterations in nuclear structure are due to a concentration-dependent dominant-negative effect of LADelta50, leading to the disruption of lamin-related functions ranging from the maintenance of nuclear shape to regulation of gene expression and DNA replication.
Figures
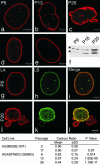
Fig. 1.
Changes in nuclear architecture as HGPS cells age in culture. Typical nuclei from HGPS cells in passages 6 (a), 13 (b), and 26 (c), normal aged human AG09602B (d), and foreskin fibroblast (e) controls after labeling with LA Ab. Nuclei in control cells appeared similar from passage 6 through passage 17. The lamina increased in prominence or thickness in HGPS cell nuclei by passage 26 (c). Earlier passage HGPS (a) and control cell nuclei (d and e) were normal in appearance. Passage 6 and 26 HGPS cells were double labeled with LA (g and j) and LB (h and k) Abs. Note the extensive coincidence in the staining patterns in the merged image at passage 6 (i) and the decrease in this coincidence in a cell by passage 26 (l). The table shows that the contour ratio decreased as a function of passage number in HGADFN003 cells. P values were calculated relative to passage 2 of the AG09602B fibroblasts. There was no significant change in the contour ratio in control cells between passages 2 and 17. In HGADFN003 whole-cell extracts immunoblotted with LA/C Abs, a band migrating between LA (upper band, *) and lamin C (lower band, *) was observed and became more prominent as the passage number increased (f). (Scale bars = 5 μm.)
Fig. 2.
Organization of nuclear pores and membranes in HGPS cells. The distribution and organization of nuclear pore complexes in a passage 6 HGPS cell (HGADFN003; a_–_c) was very similar to control AG09602B cells (not shown), as determined by double-label indirect immunofluorescence with LA and mAb 414 Abs. The typical punctate nuclear pore staining pattern along the nuclear envelope is evident. In the misshapen nuclei typifying passage 24 (d and e), the nuclear pores were frequently aggregated into large masses as detected with NUP153 Ab. Comparison of enlarged views of small regions (c and f) showing that the pores were not uniformly distributed in the nuclei of passage 26 HGPS cells (f); bracket in f shows an area that was essentially free of nucleoporins as detected by mAb414. In HGADFN003 cells stained with emerin Ab, emerin remained associated with the nuclear envelope throughout all passages (g and h). Emerin distribution appeared normal in control AG09602 cells at all passages (i). [Scale bars = 5 μm(b, e, and g_–_i) and 1 μm(c and f).]
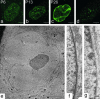
Fig. 3.
Pre-LA accumulation in the nuclei of HGPS cells. In passage 6 and 13 HGPS cells, the pre-LA pattern consisted of weakly staining nucleoplasmic foci (a and b) similar to control AG09602B fibroblasts (d). However, by passage 26, the pre-LA staining was much more intense in the lobulated nuclei and was primarily associated with the lamina region (c). (e_–_g) Electron microscopic observations of passage 26 HGPS HGADFN003 cells (e and f) and normal human foreskin fibroblasts (g). A high-magnification view of the nuclear envelope in a normal human foreskin fibroblast showed a normal array of heterochromatin adjacent to the nuclear envelope, making any lamina structure difficult to detect (g). A low-magnification view of a passage 26 HGADFN003 nucleus showed extensive lobulation (e). A higher-magnification view of a passage 26 cell showed a loss of peripheral heterochromatin and a prominent electron-dense lamina region associated with the inner nuclear envelope membrane (f). In f and g, the nucleus is to the left. [Scale bars = 5 μm (a_–_e) and 200 nm (f and g).]
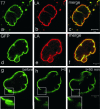
Fig. 4.
The expression of LAΔ50 in normal cells causes rapid changes in nuclear shape. (a_–_c) A control AG09602 fibroblast double-labeled with anti-T7 tag and anti-LA 35 min after the microinjection of T7-tagged LAΔ50 into its cytoplasm. Before the injection of the live cell, phase-contrast microscopy revealed that the nucleus had a smooth surface and a normal ellipsoidal shape (not shown). (d and e) A highly lobulated nucleus of a HeLa cell 48 h after transfection with pEGFP-LA-Δ150 (d) and fixation and processing for indirect immunofluorescence with anti-LA (e). The overlay seen in f demonstrated that the endogenous LA and GFP-LAΔ50 showed extensive overlap. FRAP in the nuclear envelope region of a live HeLa cell after transfection with pEGFP-LAΔ150 showed that there was no detectable recovery after 90 min (g_–_i). The box in g indicates the area of interest just before photobleaching and is shown at higher magnification in the Inset. This area is shown immediately after bleaching (h) and at 90 min after bleaching (i). (Scale bars = 5 μm.)
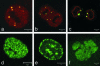
Fig. 5.
The effects of the HGPS mutation on different stages of the cell cycle. Mitotic HeLa cells expressing GFP-LAΔ50 were fixed and stained for immunofluorescence with anti-LA (red). The GFP N-terminal tag appeared green. (a) A cell in the metaphase–anaphase transition showing that LA was diffusely organized throughout the cytoplasm because of its normal disassembly during mitosis, whereas GFP-LAΔ50 was seen mainly in large cytoplasmic aggregates. (b and c) The same types of aggregates remained in cells in telophase/early cytokinesis (b) and after daughter cell nuclei had reformed in early G1 (c). (d_–_f) HGADFN003 cells processed for immunofluorescence with an Ab directed against proliferating cell nuclear antigen. Passage 6 cells with the typical pattern were seen in early (d) or mid-late (e) S phase, whereas f depicts the only pattern detected in a passage 26 cell containing a lobulated nucleus. This latter staining appeared similar to an early S-phase pattern. (Scale bars = 5 μm.)
Similar articles
- Hutchinson-Gilford progeria mutant lamin A primarily targets human vascular cells as detected by an anti-Lamin A G608G antibody.
McClintock D, Gordon LB, Djabali K. McClintock D, et al. Proc Natl Acad Sci U S A. 2006 Feb 14;103(7):2154-9. doi: 10.1073/pnas.0511133103. Epub 2006 Feb 6. Proc Natl Acad Sci U S A. 2006. PMID: 16461887 Free PMC article. - Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome.
Scaffidi P, Misteli T. Scaffidi P, et al. Nat Med. 2005 Apr;11(4):440-5. doi: 10.1038/nm1204. Epub 2005 Mar 6. Nat Med. 2005. PMID: 15750600 Free PMC article. - Progerin impairs chromosome maintenance by depleting CENP-F from metaphase kinetochores in Hutchinson-Gilford progeria fibroblasts.
Eisch V, Lu X, Gabriel D, Djabali K. Eisch V, et al. Oncotarget. 2016 Apr 26;7(17):24700-18. doi: 10.18632/oncotarget.8267. Oncotarget. 2016. PMID: 27015553 Free PMC article. - Hutchinson-Gilford progeria syndrome through the lens of transcription.
Prokocimer M, Barkan R, Gruenbaum Y. Prokocimer M, et al. Aging Cell. 2013 Aug;12(4):533-43. doi: 10.1111/acel.12070. Epub 2013 Apr 19. Aging Cell. 2013. PMID: 23496208 Review. - Epigenetic involvement in Hutchinson-Gilford progeria syndrome: a mini-review.
Arancio W, Pizzolanti G, Genovese SI, Pitrone M, Giordano C. Arancio W, et al. Gerontology. 2014;60(3):197-203. doi: 10.1159/000357206. Epub 2014 Feb 28. Gerontology. 2014. PMID: 24603298 Review.
Cited by
- Emerging Roles of 1D Vertical Nanostructures in Orchestrating Immune Cell Functions.
Chen Y, Wang J, Li X, Hu N, Voelcker NH, Xie X, Elnathan R. Chen Y, et al. Adv Mater. 2020 Oct;32(40):e2001668. doi: 10.1002/adma.202001668. Epub 2020 Aug 26. Adv Mater. 2020. PMID: 32844502 Free PMC article. Review. - AKT1-mediated Lamin A/C degradation is required for nuclear degradation and normal epidermal terminal differentiation.
Naeem AS, Zhu Y, Di WL, Marmiroli S, O'Shaughnessy RF. Naeem AS, et al. Cell Death Differ. 2015 Dec;22(12):2123-32. doi: 10.1038/cdd.2015.62. Epub 2015 Jun 5. Cell Death Differ. 2015. PMID: 26045045 Free PMC article. - Vascular senescence in progeria: role of endothelial dysfunction.
Xu Q, Mojiri A, Boulahouache L, Morales E, Walther BK, Cooke JP. Xu Q, et al. Eur Heart J Open. 2022 Jul 28;2(4):oeac047. doi: 10.1093/ehjopen/oeac047. eCollection 2022 Jul. Eur Heart J Open. 2022. PMID: 36117952 Free PMC article. - The Broad Spectrum of LMNA Cardiac Diseases: From Molecular Mechanisms to Clinical Phenotype.
Crasto S, My I, Di Pasquale E. Crasto S, et al. Front Physiol. 2020 Jul 3;11:761. doi: 10.3389/fphys.2020.00761. eCollection 2020. Front Physiol. 2020. PMID: 32719615 Free PMC article. Review. - DNA repair-related genes and adipogenesis: Lessons from congenital lipodystrophies.
Campos JTAM, Oliveira MS, Soares LP, Medeiros KA, Campos LRDS, Lima JG. Campos JTAM, et al. Genet Mol Biol. 2022 Nov 7;45(3 Suppl 1):e20220086. doi: 10.1590/1678-4685-GMB-2022-0086. eCollection 2022. Genet Mol Biol. 2022. PMID: 36354755 Free PMC article.
References
- Gilford, M. (1904) Practitioner 73, 188–217.
- DeBusk, F. L. (1972) J. Pediatr. 80, 697–724. - PubMed
- Baker, P. B., Baba, N. & Boesel, C. P. (1981) Arch. Pathol. Lab. Med. 105, 384–386. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials