Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents - PubMed (original) (raw)
Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents
Chris T Bond et al. J Neurosci. 2004.
Abstract
Action potentials in many central neurons are followed by a prolonged afterhyperpolarization (AHP) that influences firing frequency and affects neuronal integration. In hippocampal CA1 pyramidal neurons, the current ascribed to the AHP (IAHP) has three kinetic components. The IfastAHP is predominantly attributable to voltage-dependent K+ channels, whereas Ca2+-dependent and voltage-independent K+channels contribute to the ImediumAHP (ImAHP) and IslowAHP (IsAHP). Apamin, which selectively suppresses a component of the mAHP, increases neuronal excitability and facilitates the induction of synaptic plasticity at Schaffer collateral synapses and hippocampal-dependent learning. The Ca2+-dependent components of the AHP have been attributed to the activity of small conductance Ca2+-activated K+ (SK) channels. Examination of transgenic mice, each lacking one of the three SK channel genes expressed in the CNS, reveals that mice without the SK2 subunit completely lack the apamin-sensitive component of the ImAHP in CA1 neurons, whereas the IsAHP is not different in any of the SK transgenic mice. In each of the transgenic lines, the expression levels of the remaining SK genes are not changed. The results demonstrate that only SK2 channels are necessary for the ImAHP, and none of the SK channels underlie the IsAHP.
Figures
Figure 1.
SK transgenic mice. A, Schematic representations of the SK1 and SK2 transgene loci; SK1 flox (top) and SK2 flox (bottom) alleles. The sizes of the exons (in base pairs) and the channel domains they encode are presented, and the sizes of the introns (in kilobase pairs) are given for those that have been determined. The inserted elements (triangles represent loxP sites), their sizes, and positions in the targeting constructs are shown below the exon-intron mosaic. B, RT-PCR analysis using total brain cDNA shows the loss of each SK mRNA in the respective knock-out mice. β-actin was amplified to approximate the relative amounts of cDNA from the different genotypes. C, Western blots of brain proteins for SK2 and SK3. The same samples (30 μg) were loaded in each lane for each of the blots. The top blot was probed with an affinity-purified polyclonal SK2 antiserum and shows the loss of SK2 in SK2 Δ_/_Δ mice. The middle blot was probed with a polyclonal SK3 antiserum and shows the loss of SK3 protein in SK3 T/T mice that had been administered dietary doxycycline (Bond et al., 2000) (see Materials and Methods). The bottom blot was probed with a polyclonal Na+-K+ ATPase antiserum and shows the relative sample loading across all lanes.
Figure 2.
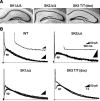
Voltage-clamp analysis of the IAHP. A, Thionin-stained hippocampal sections from SK knock-out mice. No obvious anatomical abnormalities were observed in any of the genotypes. B, Voltage-clamp recordings of mouse CA1 neurons after a 100 msec depolarizing pulse to +20 mV. Apamin (ap) blocks an initial component of the IAHP in all of the genotypes except SK2 Δ_/Δ mice, which lack the apamin-sensitive current. Subtraction of the traces before and after apamin application yielded the ImAHP (shown for wild type and SK2 Δ/Δ). Scale bars for full traces correspond to those for SK3 T/T (dox); those for the insets correspond to the values shown for SK2 Δ/_Δ.
Figure 3.
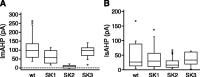
SK2 channels underlie the apamin-sensitive component of the ImAHP, whereas the SK channels do not contribute to the IsAHP. Left, Box plot of the amplitudes of the apamin-sensitive component of the ImAHP from the different genotypes measured 100 msec after the pulse. Mice lacking SK2 channels do not express an apamin-sensitive ImAHP. Statistical differences were determined by ANOVA, yielding p values <0.001 for _SK2_ Δ_/_Δ, 0.23 for _SK1_ Δ_/_Δ, 0.92 for _SK3 T/T_ (dox) mice compared with wild type. Right, Box plot of IsAHP amplitudes. The IsAHP was measured in the presence of apamin as the current amplitude at 1 sec after the end of the pulse. Statistical differences were determined by ANOVA, yielding _p_ values of 0.26 for SK2 Δ/Δ, 1.00 for _SK1_ Δ_/_Δ, 0.90 for _SK3 T/T_ (dox) mice compared with wild type. There were no significant differences between groups (_n_ > 7), showing that none of the SK channels contribute to the IsAHP.
Figure 4.
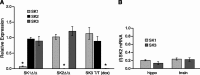
Quantitative analysis of SK mRNA levels in SK transgenic mice. A, Real-time PCR performed with whole-brain RNA from the different SK transgenic mice was used to evaluate the levels of each of the SKm RNAs, normalized to the levels determined for wild type (n = 5 for each genotype; see Materials and Methods). 18S ribosomal RNA was used as the internal reference. B, Comparison of levels of SK1 and SK3 mRNAs to SK2 mRNA in whole brain and hippocampus of wild-type mice by real-time PCR. 18S rRNA was used as the internal reference. Relative expression compared with wild type was calculated as the mean of 2-ΔΔCt ± SEM and shows that mRNA levels derived from the unmodified SK genes are not significantly altered in any of the transgenic mice. Statistically significant differences (p < 0.05) in expression levels compared with wild type are indicated by an asterisk.
Similar articles
- Small-conductance Ca2+-activated K+ channel type 2 (SK2) modulates hippocampal learning, memory, and synaptic plasticity.
Hammond RS, Bond CT, Strassmaier T, Ngo-Anh TJ, Adelman JP, Maylie J, Stackman RW. Hammond RS, et al. J Neurosci. 2006 Feb 8;26(6):1844-53. doi: 10.1523/JNEUROSCI.4106-05.2006. J Neurosci. 2006. PMID: 16467533 Free PMC article. - Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding.
Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, Adelman JP, Tzounopoulos T. Stackman RW, et al. J Neurosci. 2002 Dec 1;22(23):10163-71. doi: 10.1523/JNEUROSCI.22-23-10163.2002. J Neurosci. 2002. PMID: 12451117 Free PMC article. - SKCa channels mediate the medium but not the slow calcium-activated afterhyperpolarization in cortical neurons.
Villalobos C, Shakkottai VG, Chandy KG, Michelhaugh SK, Andrade R. Villalobos C, et al. J Neurosci. 2004 Apr 7;24(14):3537-42. doi: 10.1523/JNEUROSCI.0380-04.2004. J Neurosci. 2004. PMID: 15071101 Free PMC article. - Small conductance Ca2+-activated K+ channels as targets of CNS drug development.
Blank T, Nijholt I, Kye MJ, Spiess J. Blank T, et al. Curr Drug Targets CNS Neurol Disord. 2004 Jun;3(3):161-7. doi: 10.2174/1568007043337472. Curr Drug Targets CNS Neurol Disord. 2004. PMID: 15180477 Review. - Small conductance Ca2+-activated K+ channels and calmodulin.
Maylie J, Bond CT, Herson PS, Lee WS, Adelman JP. Maylie J, et al. J Physiol. 2004 Jan 15;554(Pt 2):255-61. doi: 10.1113/jphysiol.2003.049072. Epub 2003 Sep 18. J Physiol. 2004. PMID: 14500775 Free PMC article. Review.
Cited by
- Apamin inhibits renal fibrosis via suppressing TGF-β1 and STAT3 signaling in vivo and in vitro.
Gwon MG, An HJ, Gu H, Kim YA, Han SM, Park KK. Gwon MG, et al. J Mol Med (Berl). 2021 Sep;99(9):1265-1277. doi: 10.1007/s00109-021-02087-x. Epub 2021 May 24. J Mol Med (Berl). 2021. PMID: 34031696 - PKA and Ube3a regulate SK2 channel trafficking to promote synaptic plasticity in hippocampus: Implications for Angelman Syndrome.
Sun J, Liu Y, Zhu G, Cato C, Hao X, Qian L, Lin W, Adhikari R, Luo Y, Baudry M, Bi X. Sun J, et al. Sci Rep. 2020 Jun 17;10(1):9824. doi: 10.1038/s41598-020-66790-4. Sci Rep. 2020. PMID: 32555345 Free PMC article. - Small-conductance calcium-activated potassium type 2 channels (SK2, KCa2.2) in human brain.
Willis M, Trieb M, Leitner I, Wietzorrek G, Marksteiner J, Knaus HG. Willis M, et al. Brain Struct Funct. 2017 Mar;222(2):973-979. doi: 10.1007/s00429-016-1258-1. Epub 2016 Jun 29. Brain Struct Funct. 2017. PMID: 27357310 Free PMC article. - Calcium signaling, excitability, and synaptic plasticity defects in a mouse model of Alzheimer's disease.
Zhang H, Liu J, Sun S, Pchitskaya E, Popugaeva E, Bezprozvanny I. Zhang H, et al. J Alzheimers Dis. 2015;45(2):561-80. doi: 10.3233/JAD-142427. J Alzheimers Dis. 2015. PMID: 25589721 Free PMC article. - An introduction to murine models of atrial fibrillation.
Riley G, Syeda F, Kirchhof P, Fabritz L. Riley G, et al. Front Physiol. 2012 Aug 3;3:296. doi: 10.3389/fphys.2012.00296. eCollection 2012. Front Physiol. 2012. PMID: 22934047 Free PMC article.
References
- Bond CT, Sprengel R, Bissonnette JM, Kaufmann WA, Pribnow D, Neelands T, Storck T, Baetscher M, Jerecic J, Maylie J, Knaus HG, Seeburg PH, Adelman JP (2000) Respiration and parturition affected by conditional overexpression of the Ca2+-activated K+ channel subunit, SK3. Science 289: 1942-1946. - PubMed
- Gerlach AC, Maylie J, Adelman JP (2004) Activation kinetics of the slow afterhyperpolarization in hippocampal CA1 neurons. Pflügers Arch 448: 187-196. - PubMed
- Ishii TM, Maylie J, Adelman JP (1997a) Determinants of apamin and d-tubocurarine block in SK potassium channels. J Biol Chem 272: 23195-23200. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous