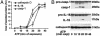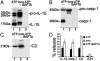Phospholipases C and A2 control lysosome-mediated IL-1 beta secretion: Implications for inflammatory processes - PubMed (original) (raw)
Phospholipases C and A2 control lysosome-mediated IL-1 beta secretion: Implications for inflammatory processes
Cristina Andrei et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Blocking the activity of IL-1 beta has entered the clinical arena of treating autoimmune diseases. However, a successful outcome of this approach requires a clear definition of the mechanisms controlling IL-1 beta release. These are still unclear as IL-1 beta, lacking a secretory signal peptide, follows a nonclassical pathway of secretion. Here, we analyze the molecular mechanism(s) undergoing IL-1 beta processing and release in human monocytes and provide a unifying model for the regulated secretion of the cytokine. Our data show that in a first step, pro-caspase-1 and endotoxin-induced pro-IL-1 beta are targeted in part to specialized secretory lysosomes, where they colocalize with other lysosomal proteins. Externalization of mature IL-1 beta and caspase-1 together with lysosomal proteins is then facilitated by extracellular ATP. ATP triggers the efflux of K(+) from the cell, followed by Ca(2+) influx and activation of three phospholipases: phosphatidylcholine-specific phospholipase C and calcium-independent and -dependent phospholipase A(2). Whereas calcium-independent phospholipase A(2) is involved in processing, phosphatidylcholine-specific phospholipase C and calcium-dependent phospholipase A(2) are required for secretion. Dissection of the events that follow ATP triggering allowed to demonstrate that K(+) efflux is responsible for phosphatidylcholine-specific phospholipase C induction, which in turn allows the rise in intracellular free calcium concentration required for activation of phospholipase A(2). This activation is ultimately responsible for lysosome exocytosis and IL-1 beta secretion.
Figures
Fig. 1.
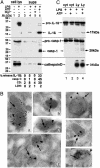
ATP induces lysosome exocytosis. (A) Monocytes untreated (lanes 1 and 3) or treated for 3 h with LPS (lanes 2, 4, and 6) or LPS plus 10 mM MgCl2 (lane 5) were incubated for 20 min without (lanes 1-5) or with (lane 6) 1 mM ATP. Cell lysates (lanes 1 and 2) and supernatants (lanes 3-6) were analyzed by Western blotting for the presence of IL-1β (Top), caspase-1 (Middle), and cathepsin D (Bottom). _M_r markers are shown on the left. The percent of release of the three proteins and of LDH is stated at the bottom. Values are expressed as percentage of total protein released after Triton X-100 lysis of untreated cells. (B) Immunoelectron microscopy analysis of caspase-1, IL-1β, lamp-1, and cathepsin D in lysosomal-enriched fractions (see Experimental Procedures). Double immunolabeling with anti-caspase-1 Ab (18-nm gold particles, arrows) and anti-IL-1β mAb (_a_-d) or anti-Lamp-1 (e and f) or anti-cathepsin D (g) (all 10-nm gold particles, arrowheads). (Bar, 100 nm.) (C) Western blotting as in A of an aliquot (1/10) of cytosolic fraction (cyt, lanes 1 and 2) and the whole endolysosomal-enriched fractions (Ly, lanes 3 and 4) from LPS-activated monocytes untreated (lanes 1 and 3) or treated for 20 min with 1 mM ATP (lanes 2 and 4). Arrows point to the mature forms of IL-1β and caspase-1 detected in lysosomes from ATP-treated monocytes only. A representative experiment of the three performed is shown.
Fig. 2.
Kinetics of IL-1β, caspase-1, and cathepsin D secretion. LPS-activated monocytes were exposed to 1 mM ATP for various times. Supernatants (A) and cell lysates (B) were analyzed by Western blotting for the presence of the three proteins. (A) Densitometric analyses of mature IL-1β, caspase-1, and cathepsin D secreted at the different times. Results are expressed as average ± SE of a single experiment performed in triplicate. One experiment of five performed is shown.
Fig. 3.
Lysosome exocytosis is calcium-dependent. LPS-activated monocytes were exposed to 1 mM ATP (lane 1) or 2 μM ionomycin (lane 2) for 20 min or preexposed to 50 μM 1,2-bis(2-aminophenoxy)ethane-tetra-acetic acid (BAPTA) for 1 h and then to ATP for 20 min (lane 3). Supernatants were analyzed for the presence of IL-1β (A), caspase-1 (B), and cathepsin D (CD) (C) by Western blot. One experiment of five performed is shown. (D) Percent of release of pro- and mature IL-1β and caspase-1, cathepsin D, and LDH ± SD.
Fig. 4.
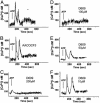
K+ regulates both IL-1β processing and lysosome exocytosis. (A) LPS-activated monocytes were exposed for 20 min to medium (lane 1) or to 1 mM ATP (lanes 2-4) in the absence (lanes 2) or in the presence of ac-YVAD-cmk (lanes 3) or of K+ buffer (KCl, lane 4). At the end of incubation, supernatants were collected and analyzed for the presence of IL-1β (Top), caspase-1 (Middle), and cathepsin D (Bottom) by Western blot. (B) Monocytes activated with LPS as in A were incubated for 30 min in Na+ buffer without ATP in the absence (lane 1) or presence (lane 2) of ac-YVAD-cmk. Supernatants were processed as in A. One representative experiment of three is shown. (C) LPS-activated monocytes were incubated in RPMI medium (Upper) or K+ buffer (Lower) supplemented with 1% FBS and challenged with 1 mM ATP (arrow), and Ca2+ waves were monitored. Results are expressed as [Ca2+]i and represent the mean of calcium response of 15 different cells.
Fig. 5.
PC-PLC and PLA2 are involved in ATP-induced lysosome exocytosis. (A) Monocytes activated 3 h with LPS alone (LPS), or followed by 2 min with 2 mM ATP, without (LPS+ATP) or with 100 μM D609 or 40 μM AACOCF3 (LPS+ATP+inhibitors), were lysed, and the presence of active PC-PLC or PLA2 was assayed. Data are expressed as fold of increase over the basal activity of PC-PLC or PLA2 in nonstimulated monocytes. Inhibition of PC-PLC activity was 55-65% with 200 and 100 μM D609 and 35-40% with 50 μM in the different experiments; inhibition of PLA2 activity was 45% with 40 μM AACOCF3 and <10% with 20 μM (data not shown). (B) LPS-activated monocytes, preincubated for 1 h in the absence or presence of different amounts of D609 (Upper) or AACOCF3 (Lower), were exposed for 20 min to 1 mM ATP. Supernatants were analyzed for the presence of IL-1β, caspase-1, or cathepsin D by Western blotting and densitometric analyses. Data are expressed as the percentage of inhibition of secretion obtained in the absence of drugs. (C) LPS-activated monocytes, preincubated for 1 h in the absence (-, lanes 1, 2, 7, and 10) or presence of 2,3-diphosphoglycerate (DPG, 10 mM, lane 3), AACOCF3 (AA, 40 μM, lanes 4, 8, and 11), D609 (200 μM, lanes 5, 9, and 12), or bromoenol lactone (BEL, 100 μM, lane 6) were exposed for 30 min to medium (lane 1) or to 1 mM ATP (lanes 2-6) or to Na+ buffer (lanes 7-9) or for 15 min to 2 μM ionomycin (lanes 10-12) in the absence or presence of the same inhibitors. Supernatants were analyzed for the presence of IL-1β (Top), caspase-1 (Middle), and cathepsin D (CD, Bottom) by Western blot. One representative experiment of five is shown.
Fig. 6.
The PC-PLC inhibitor D609, but not the PLA2 inhibitor AACOCF3, prevents the ATP-induced [Ca2+]i rise. LPS-activated monocytes untreated (A) or pretreated for 30 min with 40 μM AACOCF3 (B)or200 μM(C), 100 μM(D), 50 μM(E), or 10 μM(F) D609 were challenged with 1 mM ATP (arrow). [Ca2+]i was monitored, and results are expressed as in the legend to Fig. 4. One representative experiment of three is shown.
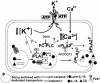
Fig. 7.
A schematic model outlining how ATP-induced PLC, iPLA2, and cPLA2 participate in IL-1β processing and secretion by LPS-activated monocytes. ATP binding causes the opening of P2X7 channels with K+ efflux and intracellular K+ depletion. This leads to activation of iPLA2, in turn responsible for generation of active caspase-1 (16), and of PC-PLC. PC-PLC activation results in [Ca2+]i rise, prevented by the PC-PLC-specific inhibitor D609. Although the underlying mechanism is unclear, PC-PLC involvement in [Ca2+]i increases has been described in other systems (42, 43). Ca2+ rise contributes with mitogen-activated protein kinase-mediated phosphorylation to activation and membrane trans-location of cPLA2 (38). Interestingly, the PC-PLC inhibitor D609 was also shown to inhibit in part mitogen-activated protein kinase activation by the 5-hydroxytryptamine receptor (44). Thus, PC-PLC may have a role also in P2X7 receptor-dependent PLA2 phosphorylation. In any case, PLA2 activation is ultimately responsible for lysosome exocytosis and release of IL-1β, caspase-1, and lysosomal enzymes.
Comment in
- IL-1beta: an endosomal exit.
Wewers MD. Wewers MD. Proc Natl Acad Sci U S A. 2004 Jul 13;101(28):10241-2. doi: 10.1073/pnas.0403971101. Epub 2004 Jul 6. Proc Natl Acad Sci U S A. 2004. PMID: 15240873 Free PMC article. Review. No abstract available.
Similar articles
- Potassium regulates IL-1 beta processing via calcium-independent phospholipase A2.
Walev I, Klein J, Husmann M, Valeva A, Strauch S, Wirtz H, Weichel O, Bhakdi S. Walev I, et al. J Immunol. 2000 May 15;164(10):5120-4. doi: 10.4049/jimmunol.164.10.5120. J Immunol. 2000. PMID: 10799869 - Mechanism of cytosol phospholipase C and sphingomyelinase-induced lysosome destabilization.
Wang X, Zhao HF, Zhang GJ. Wang X, et al. Biochimie. 2006 Jul;88(7):913-22. doi: 10.1016/j.biochi.2006.02.005. Epub 2006 Mar 20. Biochimie. 2006. PMID: 16580116 - Evolution, role in inflammation, and redox control of leaderless secretory proteins.
Sitia R, Rubartelli A. Sitia R, et al. J Biol Chem. 2020 May 29;295(22):7799-7811. doi: 10.1074/jbc.REV119.008907. Epub 2020 Apr 24. J Biol Chem. 2020. PMID: 32332096 Free PMC article. Review. - Effects of extracellular ATP on phosphatidylcholine phospholipase signaling systems.
Exton JH. Exton JH. Ann N Y Acad Sci. 1990;603:246-54; discussion 254-5. doi: 10.1111/j.1749-6632.1990.tb37676.x. Ann N Y Acad Sci. 1990. PMID: 2291524 Review. No abstract available.
Cited by
- Herpes simplex virus 1 infection induces activation and subsequent inhibition of the IFI16 and NLRP3 inflammasomes.
Johnson KE, Chikoti L, Chandran B. Johnson KE, et al. J Virol. 2013 May;87(9):5005-18. doi: 10.1128/JVI.00082-13. Epub 2013 Feb 20. J Virol. 2013. PMID: 23427152 Free PMC article. - Future perspective: high-throughput construction of new ultrasensitive cytokine and virion liquid chips for high-throughput screening (HTS) of anti-inflammatory drugs or clinical diagnosis and treatment of inflammatory diseases.
Feng Y, Huang J, Qu C, Huang M, Chen Z, Tang D, Xu Z, Wang B, Chen Z. Feng Y, et al. Anal Bioanal Chem. 2020 Nov;412(28):7685-7699. doi: 10.1007/s00216-020-02894-0. Epub 2020 Sep 1. Anal Bioanal Chem. 2020. PMID: 32870351 Free PMC article. - CAY10593 inhibits the human P2X7 receptor independently of phospholipase D1 stimulation.
Pupovac A, Stokes L, Sluyter R. Pupovac A, et al. Purinergic Signal. 2013 Dec;9(4):609-19. doi: 10.1007/s11302-013-9371-6. Epub 2013 Jun 21. Purinergic Signal. 2013. PMID: 23793974 Free PMC article. - Rab39a binds caspase-1 and is required for caspase-1-dependent interleukin-1beta secretion.
Becker CE, Creagh EM, O'Neill LA. Becker CE, et al. J Biol Chem. 2009 Dec 11;284(50):34531-7. doi: 10.1074/jbc.M109.046102. Epub 2009 Oct 15. J Biol Chem. 2009. PMID: 19833722 Free PMC article. - Blocking IL-1 in systemic inflammation.
Dinarello CA. Dinarello CA. J Exp Med. 2005 May 2;201(9):1355-9. doi: 10.1084/jem.20050640. J Exp Med. 2005. PMID: 15867089 Free PMC article. Review.
References
- Dinarello, C. A. (1998) Ann. N.Y. Acad. Sci. 856, 1-11. - PubMed
- Rubartelli, A. & Sitia, R. (1997) in Unusual Secretory Pathways: From Bacteria to Man, eds. Kuchler, K., Rubartelli, A. & Holland, B. I. (RG Landes, Austin, TX), pp. 87-104.
- Burns, K., Martinon, F. & Tschopp, J. (2003) Curr. Opin. Immunol. 15, 26-30. - PubMed
- Perregaux, D. & Gabel. C. A. (1994) J. Biol. Chem. 269, 15195-15203. - PubMed
- Ferrari, D., Chiozzi, P., Falzoni, S., Dal Susino, M., Melchiorri, L., Baricordi, O. R. & Di Virgilio, F. (1997) J. Immunol. 159, 1451-1458. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous