Cbl-dependent ubiquitination is required for progression of EGF receptors into clathrin-coated pits - PubMed (original) (raw)
Cbl-dependent ubiquitination is required for progression of EGF receptors into clathrin-coated pits
Espen Stang et al. Mol Biol Cell. 2004 Aug.
Abstract
Ligand binding causes the EGF receptor (EGFR) to become ubiquitinated by Cbl upon association with the adaptor protein Grb2. We have investigated the role of ubiquitin and Grb2 in ligand-induced endocytosis of the EGFR. Incubation of cells with EGF on ice caused translocation of Grb2 and Cbl from the cytosol to the rim of coated pits. Grb2 with point mutations in both SH3 domains inhibited recruitment of the EGFR to clathrin-coated pits, in a Ras-independent manner. On overexpression of the Cbl-binding protein Sprouty, ubiquitination of the EGFR was inhibited, the EGFR was recruited only to the rim of coated pits, and endocytosis of the EGFR was inhibited. Conjugation-defective ubiquitin similarly inhibited recruitment of EGF-EGFR to clathrin-coated pits. Even though this does not prove that cargo must be ubiquitinated, this indicates the importance of interaction of ubiquitinated protein(s) with proteins harboring ubiquitin-interacting domains. We propose that Grb2 mediates transient anchoring of the EGFR to an Eps15-containing molecular complex at the rim of coated pits and that Cbl-induced ubiquitination of the EGFR allows relocation of EGFR from the rim to the center of clathrin-coated pits.
Figures
Figure 1.
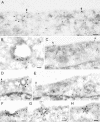
Recruitment of EGF-EGFR to clathrin-coated plasma membrane areas upon incubation with EGF on ice. Serum-starved Hep2 cells, incubated with EGF (60 ng/ml) on ice for 60 min, were prepared for immuno-EM and labeled for EGFR (A) or EGF (B and C). Labeling for EGFR and EGF (arrows) was found both at smooth and coated plasma membrane areas (coat indicated by arrowheads). That the coated areas represent clathrin-coated domains was confirmed by double-labeling for EGFR (large gold particles) and clathrin (small gold particles) shown as inset in A or EGF (large gold particles) and clathrin (small gold particles; inset in C). Although most of the EGF-EGFR positive coated areas were flat, some labeling localized to invaginated coated pits (inset in B). Bars, 100 nm.
Figure 2.
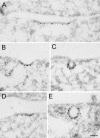
Grb2 with mutations in both SH3 domains (d.n. Grb2) inhibits endocytosis of EGF, but not Tf. (A) Hep2 cells transiently transfected with d.n. Grb2 were incubated with EGF (60 ng/ml) for 60 min on ice and prepared for immuno-EM. Thawed cryosections were double-labeled for EGFR (15-nm gold particles) and d.n. Grb2 (rabbit anti-Myc; 10-nm gold particles). Labeling for EGFR (large arrows) was restricted to smooth plasma membrane areas, no labeling was found in flat or invaginated coated plasma membrane areas (coat indicated by small arrows). (B–H) HeLa cells transiently transfected with d.n. Grb2 were incubated with EGF (60 ng/ml) for 10 min at 37°C before preparation for immuno-EM. Thawed cryosections were double-labeled for EGF (B–E) or TfR (F–H; 15-nm gold particles) and d.n. Grb2 (mouse anti-Myc; 5-nm gold particles, arrowheads). In nontransfected cells (B) the EGF-EGFR complex was efficiently endocytosed, and labeling for EGF was found in multivesicular endosomes. In transfected cells (C–H) labeling for EGF (large arrows) was restricted to smooth areas of the plasma membrane (C–E). No labeling was found in coated pits (D) or in endosomes (E). Endocytosis of TfR (F–H) was not inhibited by overexpression of d.n. Grb2, and labeling for TfR was found both in shallow (F) and fully invaginated coated pits (G) as well as in coated vesicles (H). Bars, 100 nm.
Figure 3.
Dominant negative Ras does not inhibit endocytosis of EGF or of Tf. HeLa cells were transiently transfected with HA-tagged H-Ras17N (d.n. Ras). (A) Cells were incubated with Alexa488-EGF (15 ng/ml) on ice for 15 min followed by a 15-min chase at 37°C (top panel) or with Alexa488-Tf (20 μg/ml) at 37°C for 20 min (bottom panel). Transfected cells were identified by fluorescence labeling using anti-HA antibody followed by secondary Rhodamine Red-X–conjugated antibodies. Bar, 10 μm. (B) Mock-transfected HeLa cells or HeLa cells transiently overexpressing H-Ras17N (transfection efficiency 30–35%) were incubated with EGF (60 ng/ml) on ice for 15 min and chased at 37°C for the indicated times. Cell lysates were subjected to SDS-PAGE and immunoblotting with antibody to phospho-MAPK (pMAPK). SDS-PAGE and immunoblotting was as previously described (Stang et al., 2000). (C) Mock-transfected HeLa cells (•) or HeLa cells transiently overexpressing H-Ras17N (▪) were incubated with 125I-EGF (1 ng/ml) on ice for 15 min and chased at 37°C for the indicated time periods. Analysis of internalized EGF was performed as described in MATERIAL AND METHODS. The data represent the mean of two independent experiments with three parallels ± SEM.
Figure 4.
Recruitment of Grb2, Cbl, and Eps15 to the plasma membrane upon incubation with EGF on ice. (A) Hep2 cells, incubated without ligand (control) or with EGF (60 ng/ml) for 60 min on ice, were prepared for immunofluorescence and labeled for Grb2, Cbl, or Eps15, respectively. On incubation with EGF on ice, Grb2, Cbl, and Eps15 were recruited to the plasma membrane. Bar, 10 μm. (B) Thawed cryosections of serum-starved Hep2 cells, incubated with EGF (60 ng/ml) on ice for 60 min, were either single-labeled for Grb2 (I) or double-labeled for Grb2 (large gold particles) and either EGF (II) or clathrin (III, IV; small gold particles). Note that although coat-associated labeling for Grb2 (large arrows in I-IV) is restricted to the rim of the coated area, labeling for EGF (small arrows in II) is all along the coat. The double-labeling for Grb2 and clathrin confirmed that Grb2 is associated both with flat and invaginated clathrin-coated plasma membrane. Sections from the same specimen were either labeled only for Cbl (V–VI), or both for Cbl (large gold particles) and clathrin (small gold particles; VII and VIII). Note that the coat associated labeling for Cbl (arrows) is restricted to the rim of the coated area. The double-labeling for Cbl and clathrin confirmed that Cbl is associated with both flat and invaginated clathrin-coated plasma membrane. Bars, 100 nm.
Figure 5.
Localization of epsin and dynamin to coated plasma membrane areas. Thawed cryosections of serum-starved Hep2 cells, incubated with EGF (60 ng/ml) on ice for 60 min, were labeled for epsin (A–C) or for dynamin (D and E). Note that both epsin and dynamin are localized all along the coated plasma membrane area, both in flat coats and in invaginated coated pits. Bar, 100 nm.
Figure 6.
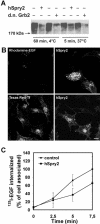
Endocytosis of EGFR, but not of TfR, is inhibited in cells where ubiquitination of the EGFR is inhibited because of overexpression of hSpry2. (A) HeLa cells transfected with HA-ubiquitin and with or without either GFP-tagged hSpry2 or d.n. Grb2 were incubated with 60 ng/ml EGF as indicated. The EGFR was immunoprecipitated, and the precipitate was subjected to Western blotting with antibody to HA. (B) HeLa cells on coverslips were transfected with GFP-tagged hSpry2 for 24 h. Rhodamine-EGF (15 ng/ml; top panel) or TexasRed-Tf (20 μg/ml; bottom panel) was added at 37°C for 15 min before the cells were fixed. Bar, 10 μm. (C) Mock-transfected HeLa cells (▪) or HeLa cells transiently overexpressing GFP-tagged hSpry2 (•) for 24 h were incubated with 125I-EGF (1 ng/ml) on ice for 15 min and chased at 37°C for the indicated time periods. Analysis of internalized EGF was performed as described in MATERIALS AND METHODS. The data represent one typical experiment with six parallels ± SD.
Figure 7.
Recruitment of EGFR into coated pits is inhibited in cells overexpressing hSpry2. HeLa cells transfected with GFP-tagged hSpry2 for 24 h were incubated with EGF (60 ng/ml) on ice for 60 min and prepared for immuno-EM. (A and B) Thawed cryosections were single-labeled using anti-GFP antibodies and 15-nm protein A gold. Labeling for GFP-tagged hSpry2 (arrows) was found both at smooth and coated plasma membrane areas (coat indicated by arrowheads). (C) Double-labeling using anti-GFP antibodies (large gold particles) followed by anti-clathrin antibodies (small gold, small arrows). (D–F) Double-labeling using either anti-EGF (D and E) or anti-EGFR (F) antibodies (large gold), followed by anti-GFP antibodies (small gold, arrows). (G) Double-labeling using anti-Grb2 antibodies (large gold) followed by anti-GFP antibodies (small gold). Bars, 100 nm.
Figure 8.
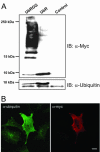
Conjugation of Myc-tagged ubiquitin to cellular proteins is blocked when the C-terminal glycine residues (75–76) are deleted. (A) HeLa cells were transfected with Myc-tagged UbRGG or Myctagged UbR for 24 h as described in MATERIALS AND METHODS. The cells were lysed, and the lysates were subjected to SDS-PAGE (10–20% Tris-Tricine) and immunoblotting with a rabbit anti-Myc antibody (top panel) or with an antibody recognizing both monomeric and conjugated ubiquitin (bottom panel). In the transfected cells two monomeric ubiquitin bands are visualized by the antibody to ubiquitin. The top band represents the Myc-tagged ubiquitin. Control = nontransfected cells. (B) HeLa cells grown on coverslips were transfected with Myc-tagged UbR for 24 h. The cells were fixed and immunostained, using a rabbit anti-Myc antibody and a mouse anti-ubiquitin antibody recognizing monomeric and conjugated ubiquitin. The secondary antibodies were Alexa488- and Rhodamine Red-X conjugated, respectively.
Figure 9.
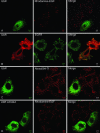
Endocytosis of EGF and EGFR is inhibited upon overexpression of ubiquitin that cannot be conjugated to endogenous proteins. Hep2 cells on coverslips were transfected with Myc-tagged UbR (A–C) or with Myc-tagged UbR L8A/I44A (D) for 48 h. Rhodamine-EGF (10 ng/ml, A and D), EGF (100 ng/ml, B), or Alexa594-Tf (50 μg/ml, C) was added to the cells at 37°C for 15 min. The cells were fixed and immunostained using mouse anti-Myc antibodies and secondary Rhodamine Red-X– or FITC-conjugated antibodies to detect transfected cells (left columns). Cells in B were additionally immunostained with anti-EGFR antibodies, followed by secondary FITC-conjugated antibodies. Bar, 5 μm.
Figure 10.
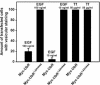
Mutant ubiquitin (UbR) inhibits endocytosis of EGF, but not of Tf. Hep2 cells were treated as described in legend to Figure 9. To quantify the uptake of EGF or Tf after 15 min, transfected cells with or without vesicular staining of Rhodamine-EGF or Alexa594-Tf were counted manually in the microscope. Transfected cells with vesicular staining are presented as percentage of the total number of transfected cells. Transfected cells with different expression of the transgene were picked randomly. For each transfection, 60 cells (20 cells each from 3 different coverslips) were analyzed. Error bars, SEM.
Figure 11.
Mutant ubiquitin (UbR) inhibits recruitment of EGF-EGFR to coated pits. Hep2 cells transiently transfected with UbR were incubated with EGF (60 ng/ml) for 60 min on ice and prepared for immuno-EM. Thawed cryosections were double-labeled for EGF (15-nm gold particles) and UbR (rabbit anti-Myc; 10-nm gold particles, indicated by small arrows in A). In transfected cells labeling for EGF was restricted to smooth plasma membrane areas (large arrowheads in A and B). A very small proportion of 15-nm gold particles (indicating EGF, arrow in B) was found in coated plasma membrane areas (coats indicated by small arrowheads). Bars, 100 nm.
Similar articles
- Activation of the epidermal growth factor (EGF) receptor induces formation of EGF receptor- and Grb2-containing clathrin-coated pits.
Johannessen LE, Pedersen NM, Pedersen KW, Madshus IH, Stang E. Johannessen LE, et al. Mol Cell Biol. 2006 Jan;26(2):389-401. doi: 10.1128/MCB.26.2.389-401.2006. Mol Cell Biol. 2006. PMID: 16382132 Free PMC article. - Both clathrin-positive and -negative coats are involved in endosomal sorting of the EGF receptor.
Myromslien FD, Grøvdal LM, Raiborg C, Stenmark H, Madshus IH, Stang E. Myromslien FD, et al. Exp Cell Res. 2006 Oct 1;312(16):3036-48. doi: 10.1016/j.yexcr.2006.06.004. Epub 2006 Jun 8. Exp Cell Res. 2006. PMID: 16859684 - Endocytosis and intracellular trafficking of ErbBs.
Sorkin A, Goh LK. Sorkin A, et al. Exp Cell Res. 2008 Oct 15;314(17):3093-106. doi: 10.1016/j.yexcr.2008.08.013. Epub 2008 Aug 28. Exp Cell Res. 2008. PMID: 18793634 Free PMC article. Review. - Endocytosis and intracellular trafficking of ErbBs.
Sorkin A, Goh LK. Sorkin A, et al. Exp Cell Res. 2009 Feb 15;315(4):683-96. doi: 10.1016/j.yexcr.2008.07.029. Exp Cell Res. 2009. PMID: 19278030 Review.
Cited by
- Impaired degradation followed by enhanced recycling of epidermal growth factor receptor caused by hypo-phosphorylation of tyrosine 1045 in RBE cells.
Gui A, Kobayashi A, Motoyama H, Kitazawa M, Takeoka M, Miyagawa S. Gui A, et al. BMC Cancer. 2012 May 16;12:179. doi: 10.1186/1471-2407-12-179. BMC Cancer. 2012. PMID: 22591401 Free PMC article. - Contact-dependent inhibition of EGFR signaling by Nf2/Merlin.
Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI. Curto M, et al. J Cell Biol. 2007 Jun 4;177(5):893-903. doi: 10.1083/jcb.200703010. J Cell Biol. 2007. PMID: 17548515 Free PMC article. - Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity.
Husebye H, Halaas Ø, Stenmark H, Tunheim G, Sandanger Ø, Bogen B, Brech A, Latz E, Espevik T. Husebye H, et al. EMBO J. 2006 Feb 22;25(4):683-92. doi: 10.1038/sj.emboj.7600991. Epub 2006 Feb 9. EMBO J. 2006. PMID: 16467847 Free PMC article. - Ubiquitin-dependent sorting in endocytosis.
Piper RC, Dikic I, Lukacs GL. Piper RC, et al. Cold Spring Harb Perspect Biol. 2014 Jan 1;6(1):a016808. doi: 10.1101/cshperspect.a016808. Cold Spring Harb Perspect Biol. 2014. PMID: 24384571 Free PMC article. Review. - APPL endosomes are not obligatory endocytic intermediates but act as stable cargo-sorting compartments.
Kalaidzidis I, Miaczynska M, Brewińska-Olchowik M, Hupalowska A, Ferguson C, Parton RG, Kalaidzidis Y, Zerial M. Kalaidzidis I, et al. J Cell Biol. 2015 Oct 12;211(1):123-44. doi: 10.1083/jcb.201311117. J Cell Biol. 2015. PMID: 26459602 Free PMC article.
References
- Aguilar, R.C., Watson, H.A., and Wendland, B. (2003). The yeast Epsin Ent1 is recruited to membranes through multiple independent interactions. J. Biol. Chem. 278, 10737-10743. - PubMed
- Amyere, M., Mettlen, M., Van Der Smissen, P., Platek, A., Payrastre, B., Veithen, A., and Courtoy, P.J. (2002). Origin, originality, functions, subversions and molecular signalling of macropinocytosis. Int. J. Med. Microbiol. 291, 487-494. - PubMed
- Barbieri, M.A., Kohn, A.D., Roth, R.A., and Stahl, P.D. (1998). Protein kinase B/akt and rab5 mediate Ras activation of endocytosis. J. Biol. Chem. 273, 19367-19370. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous