Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes - PubMed (original) (raw)
Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes
Melissa W Boulé et al. J Exp Med. 2004.
Abstract
Dendritic cell (DC) activation by nucleic acid-containing immunoglobulin (Ig)G complexes has been implicated in systemic lupus erythematosus (SLE) pathogenesis. However, the mechanisms responsible for activation and subsequent disease induction are not completely understood. Here we show that murine DCs are much more effectively activated by immune complexes that contain IgG bound to chromatin than by immune complexes that contain foreign protein. Activation by these chromatin immune complexes occurs by two distinct pathways. One pathway involves dual engagement of the Fc receptor FcgammaRIII and Toll-like receptor (TLR)9, whereas the other is TLR9 independent. Furthermore, there is a characteristic cytokine profile elicited by the chromatin immune complexes that distinguishes this response from that of conventional TLR ligands, notably the induction of BAFF and the lack of induction of interleukin 12. The data establish a critical role for self-antigen in DC activation and explain how the innate immune system might drive the adaptive immune response in SLE.
Figures
Figure 1.
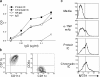
Chromatin IC and protein IC bind DCs comparably. (a) Protein IC (anti-TNP IgG2a mAb plus TNP-BSA) and chromatin IC (antinucleosome IgG2a mAb plus spent culture fluid) were tested for immune complex formation in a C1q binding assay and compared with monomeric or heat-aggregated (HA) mouse IgG. (b) FACS® analysis of bone marrow–derived DCs from wild-type C57BL/6 mice purified with anti-CD11c magnetic beads. (c) The ability of the monomeric anti-TNP mAb (α-TNP mAb) and the immune complexes described above to bind to bone marrow–derived DCs from wild-type C57BL/6 mice was compared by measuring the amount of IgG2a bound to the cell surface using flow cytometry.
Figure 2.
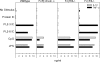
Chromatin IC, but not protein IC, induce TNF-α production. Bone marrow–derived DCs from wild-type C57BL/6 mice were preincubated for 30 min with or without (a) 12 μg/ml inhibitory CpG sODN 2088, (b) sODN 2138, sODN 1982, sODN 2088 (all at 1 μg/ml), and (c) 20 μg/ml chloroquine before the addition of 50 μg/ml protein IC, 50 μg/ml chromatin IC (PL2-3, IgG2a; PL2-8, IgG2b), 6 μg/ml of the stimulatory CpG sODN 1826, 10 μg/ml LPS, and 100 μg/ml poly(I:C). TNF-α and IL-12 p70 concentrations in supernatants collected after 48 h were determined by ELISA. The data are representative of nine (a), three (b), and three experiments (c).
Figure 3.
Chromatin IC–induced TNF-α production involves MyD88 and TLR9. Bone marrow–derived DCs from wild-type C57BL/6 and MyD88-deficient mice (left), or wild-type BALB/c and TLR9-deficient mice (right), were cultured with protein IC, chromatin IC (PL2-3, IgG2a; PL2-8, IgG2b), the stimulatory CpG sODN 1826, and LPS. TNF-α concentrations in supernatants collected after 48 h were determined by ELISA. Data represent mean + SEM of three (left) and five experiments (right).
Figure 4.
FcγRIII is required for chromatin IC–induced TNF-α production. Bone marrow–derived DCs from wild-type C57BL/6, Fc receptor common γ chain–deficient (FcRγ chain −/−), FcγRIII-deficient (FcγRIII−/−), and FcγRII-deficient (FcγRII−/−) mice were cultured with protein IC, chromatin IC (PL2-3, IgG2a; PL2-8, IgG2b), the stimulatory CpG sODN 1826, and LPS. TNF-α and IL-12 p70 concentrations in supernatants collected after 48 h were determined by ELISA. The data are representative of three experiments.
Figure 5.
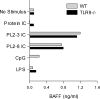
BAFF induction by chromatin IC is TLR9 independent. Bone marrow–derived DCs from wild-type BALB/c and TLR9-deficient mice were cultured with protein IC, chromatin IC (PL2-3, IgG2a; PL2-8, IgG2b), the stimulatory CpG sODN 1826, and LPS. BAFF concentration in supernatants collected after 48 h was determined by ELISA. The data are representative of three experiments.
Figure 6.
Nucleosome/antinucleosome complexes activate DCs. (a) Bone marrow–derived DCs from wild-type BALB/c and TLR9-deficient mice were cultured with the stimulatory CpG sODN 1826, LPS, R848, nucleosome fraction 4 (nucleosome 4; mainly mononucleosomes), nucleosome fraction 7 (nucleosome 7; mainly di-nucleosomes and tri-nucleosomes), the antinucleosome IgG2a mAb PL2-3, PL2-3 and nucleosome 4, PL2-3 and nucleosome 7, protein IC (anti-TNP IgG2a mAb plus TNP-BSA), and protein IC and nucleosome 4. TNF-α concentrations in supernatants collected after 24 h were determined by ELISA. The data are representative of three experiments. (b) Day 6 bone marrow–derived DC cultures (not CD11c purified) from wild-type BALB/c and TLR9-deficient mice were cultured with stimuli as shown. After 24 h of incubation, DCs were double stained with anti–CD11c-PE and anti–CD86-FITC. CD86 expression of the CD11c+ population gate is shown with stimulus-induced staining intensity (black) compared with staining intensity of the nonstimulated cultures (gray). The data are representative of three experiments.
Similar articles
- Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors.
Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Leadbetter EA, et al. Nature. 2002 Apr 11;416(6881):603-7. doi: 10.1038/416603a. Nature. 2002. PMID: 11948342 - Tamm-Horsfall glycoprotein links innate immune cell activation with adaptive immunity via a Toll-like receptor-4-dependent mechanism.
Säemann MD, Weichhart T, Zeyda M, Staffler G, Schunn M, Stuhlmeier KM, Sobanov Y, Stulnig TM, Akira S, von Gabain A, von Ahsen U, Hörl WH, Zlabinger GJ. Säemann MD, et al. J Clin Invest. 2005 Feb;115(2):468-75. doi: 10.1172/JCI22720. J Clin Invest. 2005. PMID: 15650774 Free PMC article. - Emerging roles of TLR7 and TLR9 in murine SLE.
Santiago-Raber ML, Baudino L, Izui S. Santiago-Raber ML, et al. J Autoimmun. 2009 Nov-Dec;33(3-4):231-8. doi: 10.1016/j.jaut.2009.10.001. Epub 2009 Oct 21. J Autoimmun. 2009. PMID: 19846276 Review. - Toll-like receptor activation in the pathogenesis of systemic lupus erythematosus.
Means TK, Luster AD. Means TK, et al. Ann N Y Acad Sci. 2005 Dec;1062:242-51. doi: 10.1196/annals.1358.027. Ann N Y Acad Sci. 2005. PMID: 16461805 Review.
Cited by
- TLR tolerance reduces IFN-alpha production despite plasmacytoid dendritic cell expansion and anti-nuclear antibodies in NZB bicongenic mice.
Pau E, Cheung YH, Loh C, Lajoie G, Wither JE. Pau E, et al. PLoS One. 2012;7(5):e36761. doi: 10.1371/journal.pone.0036761. Epub 2012 May 4. PLoS One. 2012. PMID: 22574220 Free PMC article. - Selectively targeting the toll-like receptor 9 (TLR9)--IRF 7 signaling pathway by polymer blend particles.
Chen HC, Zhan X, Tran KK, Shen H. Chen HC, et al. Biomaterials. 2013 Sep;34(27):6464-72. doi: 10.1016/j.biomaterials.2013.05.016. Epub 2013 Jun 5. Biomaterials. 2013. PMID: 23755833 Free PMC article. - Deoxyribonuclease 1-Mediated Clearance of Circulating Chromatin Prevents From Immune Cell Activation and Pro-inflammatory Cytokine Production, a Phenomenon Amplified by Low Trap1 Activity: Consequences for Systemic Lupus Erythematosus.
Felux J, Erbacher A, Breckler M, Hervé R, Lemeiter D, Mannherz HG, Napirei M, Rammensee HG, Decker P. Felux J, et al. Front Immunol. 2021 Mar 4;12:613597. doi: 10.3389/fimmu.2021.613597. eCollection 2021. Front Immunol. 2021. PMID: 33746957 Free PMC article. - Tumor necrosis: A synergistic consequence of metabolic stress and inflammation.
Yee PP, Li W. Yee PP, et al. Bioessays. 2021 Jul;43(7):e2100029. doi: 10.1002/bies.202100029. Epub 2021 May 16. Bioessays. 2021. PMID: 33998010 Free PMC article. - Toll/interleukin-1 receptor domain dimers as the platform for activation and enhanced inhibition of Toll-like receptor signaling.
Fekonja O, Benčina M, Jerala R. Fekonja O, et al. J Biol Chem. 2012 Sep 7;287(37):30993-1002. doi: 10.1074/jbc.M112.376186. Epub 2012 Jul 24. J Biol Chem. 2012. PMID: 22829600 Free PMC article.
References
- Kotzin, B.L. 1996. Systemic lupus erythematosus. Cell. 85:303–306. - PubMed
- Botto, M., C. Dell'Agnola, A.E. Bygrave, E.M. Thompson, H.T. Cook, F. Petry, M. Loos, P.P. Pandolfi, and M.J. Walport. 1998. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 19:56–59. - PubMed
- Napirei, M., H. Karsunky, B. Zevnik, H. Stephan, H.G. Mannherz, and T. Moroy. 2000. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat. Genet. 25:177–181. - PubMed
- Bickerstaff, M.C.M., M. Botto, W.L. Hutchinson, J. Herbert, G.A. Tennent, A. Bybee, D.A. Mitchell, H.T. Cook, P.J.G. Butler, M.J. Walport, et al. 1999. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nat. Med. 5:694–697. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases