Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures - PubMed (original) (raw)
Comparative Study
Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures
Nadia M Tsankova et al. J Neurosci. 2004.
Abstract
The mechanism of action of electroconvulsive seizures (ECS), one of the most effective treatments of major depression, may involve the regulation of gene expression. Chromatin remodeling at gene promoter regions is increasingly recognized as a key control point of gene expression and may, therefore, partly mediate acute and chronic effects of ECS on gene activity. Here, we assayed how posttranslational modifications of histones, a major form of chromatin remodeling, are altered at several gene promoters in rat hippocampus at 30 min, 2 hr, and 24 hr after acute or repeated ECS. We performed chromatin immunoprecipitation assays to measure levels of histone H3 and H4 acetylation and phosphoacetylation at the promoters of the c-fos, BDNF, and CREB (cAMP response element-binding protein) genes, the expression of which is altered by ECS. We found that, with few exceptions, levels of H4 acetylation correlated with mRNA levels for c-fos, BDNF, and CREB throughout the acute and chronic time course study, whereas acetylation and phosphoacetylation of H3 were detected more selectively. Our findings suggest that the chronic downregulation of c-fos transcription, observed in this study, may be achieved at the level of H4 acetylation, whereas chronic upregulation of BDNF transcription may be sustained via control of H3 acetylation, selectively at the BDNF P3 and P4 promoters. These data provide the first in vivo demonstration of the involvement of chromatin remodeling in ECS-induced regulation of gene expression in the brain and will help in understanding the mechanisms underlying the efficacy of ECS in the treatment of depression.
Figures
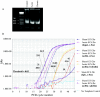
Figure 1.
Representative steps in the chromatin immunoprecipitation assay used to measure histone modifications in the hippocampus of ECS-treated and control rats. A, Chromatin was sonicated to fragments of 400-600 bp in size and run on a 2% agarose gel. Ethidium bromide staining of a resulting, representative gel is shown. B, Levels of acetylated H4 at various gene promoters in the hippocampus were quantified using real-time PCR, by comparing relative Ct values for the various genes of interest at a threshold of 0.02. (Parallel experiments, not shown, were performed for acetylated and phosphoacetylated H3.) Ct values of immunoprecipitated samples (Control and Acute ECS-2 hr with antibody specific for acetylated H4 and PCR amplified at the c-fos gene promoter) were normalized to Ct values obtained from “Input,” or non-immunoprecipitated genomic DNA, at which there is no difference between acute ECS and control samples, as expected. Comparison of these Ct values revealed a 3.5-fold increase in levels of H4 acetylation at the c-fos gene in acute ECS versus control (Sham ECS) samples. In aliquots of the same samples, Ct values from theβ_-tubulin_ gene differed by a negligible 0.1, reflecting no regulation by acute ECS. Immunoprecipitation with nonimmune IgG yielded much higher Ct values, typically 16 cycles higher, indicating a ∼65,000-fold difference (i.e., negligible precipitation of the c-fos gene in the absence of specific antibody). Finally, analysis of ϵ-globin, a gene that is silenced in adult animals and contained within transcriptionally inactive heterochromatin, reveals virtually nondetectable levels of H4 acetylation.
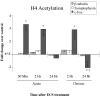
Figure 2.
Regulation of H4 acetylation at gene promoters after acute or chronic ECS. Levels of H4 acetylation at the synaptophysin and β_-tubulin_ promoters, two genes with expression that is not regulated by ECS, are compared with the levels of H4 acetylation at the c-fos promoter at 30 min, 2 hr, and 24 hr after acute or chronic ECS. Note the robust, time-dependent regulation of H4 acetylation at the c-fos promoter, but lack of regulation at the synaptophysin and β_-tubulin_ promoters, under these conditions. Data are expressed as means ± SEM (n = 4-6 in each treatment group). Control (sham-ECS) values are 1± the following SEMs: 0.02 (c-fos, 30 min), 0.05 (c-fos, 2 hr), 0.04 (c-fos, 24 hr), 0.04 (β_-tubulin_, 2 hr), 0.06 (β_-tubulin_, 24 hr), 0.05 (synaptophysin, 30 min), and 0.03 (synaptophysin, 2 hr). *p < 0.05, different from control; †p < 0.05, different from acute ECS-24 hr, by t test.
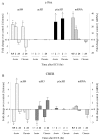
Figure 3.
Patterns of histone modifications after acute orchronic ECS at the c-fos and CREB promoters. Levels of mRNA for these genes were measured as well. Changes in histone modifications and mRNA are represented as mean ± SEM of fold change over control (control, 1 ± SEM; n = 4-6; *p < 0.05 from control). Values with large variability (in Table 1, indicated by d) are not depicted; these data points (data not shown) correspond to the time points written in gray. Table 1 presents all of the data in quantitative form. A, Changes at the c-fos promoter. At 30 min after an acute ECS, there was a 3.3-fold increase in H4 acetylation (acH4) and a 32.4-fold increase in levels of c-fos mRNA. At 2 hr after acute ECS, there was a threefold increase in H4 acetylation, a 3.7-fold increase in H3 phosphoacetylation (p/acH3), and a 15.1-fold increase in mRNA levels. At 24 hr after acute ECS, there were no significant changes in histone modifications or c-fos mRNA levels. After chronic ECS, the same histone modifications were observed at 2 hr as seen in the acute situation. However, at 24 hr, chronic ECS caused a twofold decrease in H4 acetylation, which correlated with a 1.8-fold decrease in levels of c-fos mRNA. B, Changes at the CREB promoter. At 30 min after an acute ECS, there was a 2.8-fold decrease in H4 acetylation, large variability in levels of H3 acetylation (acH3) and phosphoacetylation, and no change in CREB mRNA levels. At 2 hr after acute ECS, there was a 4.9-fold increase in H4 acetylation acutely, but a 2.7-fold decrease in H3 acetylation. CREB mRNA levels showed a trend toward a small (1.5-fold) increase. At 24 hr after acute ECS, no significant changes in histone modifications or CREB mRNA levels were observed. After chronic ECS, the major differences from the acute situation were seen at 24 hr, when a 2.5-fold decrease in H4 acetylation but a 2.4-fold increase in H3 acetylation were observed along with a 1.8-fold decrease in CREB mRNA levels.
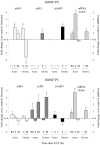
Figure 4.
Patterns of histone modifications after acute or chronic ECS at the BDNF P2 and P3 promoters. At 30 min after an acute ECS, no significant changes in histone modifications were observed at the P2 or P3 promoter, whereas total BDNF mRNA levels were increased by 1.8-fold. At 2 hr after acute ECS, H4 acetylation increased 2.8-fold at the P2 promoter, with no changes seen in H3 at either the P2 or P3 promoters, whereas total BDNF mRNA levels increased by 4.8-fold. At 24 hr after acute ECS, when BDNF mRNA levels had returned to control values, levels of H4 acetylation were increased 1.9-fold at the P2 promoter, whereas H3 acetylation tended to increase at the P3 promoter. After chronic ECS, the major differences from the acute situation were seen at 24 hr, when a 3.6-fold decrease in H4 acetylation at the P2 promoter but a 4.1-fold increase in H3 acetylation at the P3 promoter were observed along with a 2.1-fold increase in total BDNF mRNA levels. In general, the P4 promoter showed similar alterations in histone modifications as seen for the P3 promoter: 2 hr Acute ECS: H4 acetylation, -336 ± 79%(*); H3 acetylation, 134 ± 31%; 24 hr Acute ECS: H4 acetylation, -202 ± 96%; H3 acetylation, 132 ± 5%; 24 hr Chronic ECS: H4 acetylation, -119 ± 19%; H3 acetylation, 184 ± 11%(*) (n = 6-8; *p < 0.05).
Similar articles
- Single immobilization stress differentially alters the expression profile of transcripts of the brain-derived neurotrophic factor (BDNF) gene and histone acetylation at its promoters in the rat hippocampus.
Fuchikami M, Morinobu S, Kurata A, Yamamoto S, Yamawaki S. Fuchikami M, et al. Int J Neuropsychopharmacol. 2009 Feb;12(1):73-82. doi: 10.1017/S1461145708008997. Epub 2008 Jun 11. Int J Neuropsychopharmacol. 2009. PMID: 18544182 - Shear stress-mediated chromatin remodeling provides molecular basis for flow-dependent regulation of gene expression.
Illi B, Nanni S, Scopece A, Farsetti A, Biglioli P, Capogrossi MC, Gaetano C. Illi B, et al. Circ Res. 2003 Jul 25;93(2):155-61. doi: 10.1161/01.RES.0000080933.82105.29. Epub 2003 Jun 12. Circ Res. 2003. PMID: 12805238 - Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum.
Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, Neve RL, Self DW, Nestler EJ. Kumar A, et al. Neuron. 2005 Oct 20;48(2):303-14. doi: 10.1016/j.neuron.2005.09.023. Neuron. 2005. PMID: 16242410 - Epigenetic landscape of amphetamine and methamphetamine addiction in rodents.
Godino A, Jayanthi S, Cadet JL. Godino A, et al. Epigenetics. 2015;10(7):574-80. doi: 10.1080/15592294.2015.1055441. Epigenetics. 2015. PMID: 26023847 Free PMC article. Review. - Histone acetylation and chromatin remodeling: which comes first?
Neely KE, Workman JL. Neely KE, et al. Mol Genet Metab. 2002 May;76(1):1-5. doi: 10.1016/s1096-7192(02)00014-8. Mol Genet Metab. 2002. PMID: 12175774 Review. No abstract available.
Cited by
- Epileptogenesis: can the science of epigenetics give us answers?
Lubin FD. Lubin FD. Epilepsy Curr. 2012 May;12(3):105-10. doi: 10.5698/1535-7511-12.3.105. Epilepsy Curr. 2012. PMID: 22690136 Free PMC article. - Epigenetic marks and their relationship with BDNF in the brain of suicide victims.
Misztak P, Pańczyszyn-Trzewik P, Nowak G, Sowa-Kućma M. Misztak P, et al. PLoS One. 2020 Sep 24;15(9):e0239335. doi: 10.1371/journal.pone.0239335. eCollection 2020. PLoS One. 2020. PMID: 32970734 Free PMC article. - Mitogen- and stress-activated protein kinase 1-induced neuroprotection in Huntington's disease: role on chromatin remodeling at the PGC-1-alpha promoter.
Martin E, Betuing S, Pagès C, Cambon K, Auregan G, Deglon N, Roze E, Caboche J. Martin E, et al. Hum Mol Genet. 2011 Jun 15;20(12):2422-34. doi: 10.1093/hmg/ddr148. Epub 2011 Apr 14. Hum Mol Genet. 2011. PMID: 21493629 Free PMC article. - p75NTR, but not proNGF, is upregulated following status epilepticus in mice.
VonDran MW, LaFrancois J, Padow VA, Friedman WJ, Scharfman HE, Milner TA, Hempstead BL. VonDran MW, et al. ASN Neuro. 2014 Sep 25;6(5):1759091414552185. doi: 10.1177/1759091414552185. Print 2014. ASN Neuro. 2014. PMID: 25290065 Free PMC article.
References
- Chakrabarti SK, James JC, Mirmira RG (2002) Quantitative assessment of gene targeting in vitro and in vivo by the pancreatic transcription factor, Pdx1. Importance of chromatin structure in directing promoter binding. J Biol Chem 277: 13286-13293. - PubMed
- Chen J, Sochivko D, Beck H, Marechal D, Wiestler OD, Becker AJ (2001) Activity-induced expression of common reference genes in individual cns neurons. Lab Invest 81: 913-916. - PubMed
- Cheung P, Tanner KG, Cheung WL, Sassone-Corsi P, Denu JM, Allis CD (2000) Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. Mol Cell 5: 905-915. - PubMed
- Clayton AL, Mahadevan LC (2003) MAP kinase-mediated phosphoacetylation of histone H3 and inducible gene regulation. FEBS Lett 546: 51-58. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources