Membrane protein turnover by the m-AAA protease in mitochondria depends on the transmembrane domains of its subunits - PubMed (original) (raw)
Membrane protein turnover by the m-AAA protease in mitochondria depends on the transmembrane domains of its subunits
Daniel Korbel et al. EMBO Rep. 2004 Jul.
Abstract
AAA proteases are membrane-bound ATP-dependent proteases that are present in eubacteria, mitochondria and chloroplasts and that can degrade membrane proteins. Recent evidence suggests dislocation of membrane-embedded substrates for proteolysis to occur in a hydrophilic environment; however, next to nothing is known about the mechanism of this process. Here, we have analysed the role of the membrane-spanning domains of Yta10 and Yta12, which are conserved subunits of the hetero-oligomeric m-AAA protease in the mitochondria of Saccharomyces cerevisiae. We demonstrate that the m-AAA protease retains proteolytic activity after deletion of the transmembrane segments of either Yta10 or Yta12. Although the mutant m-AAA protease is still capable of processing cytochrome c peroxidase and degrading a peripheral membrane protein, proteolysis of integral membrane proteins is impaired. We therefore propose that transmembrane segments of m-AAA protease subunits have a direct role in the dislocation of membrane-embedded substrates.
Figures
Figure 1
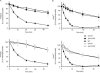
Restoration of respiratory growth of Δ_yta10_ and Δ_yta12_ cells on expression of Yta10ΔTM or Yta12ΔTM, respectively. (A–C) Cells were grown at 30°C on selective media containing the required auxotrophs and glucose (2%) as the sole carbon source. Fivefold serial dilutions of logarithmically growing cultures were spotted onto yeast peptone medium containing glycerol (3%) and incubated for 2 days at 30°C. (D) Co-immunoprecipitation of Yta12 with Yta10ΔTM. Mitochondria isolated from wild-type (WT), Δ_yta10_ and yta10_Δ_TM cells were solubilized in digitonin and incubated with Yta10specific antibodies. The immunoprecipitate was analysed by SDS–PAGE and immunoblotting using Yta12-specific antiserum.
Figure 2
Respiratory competence of yta10_Δ_TM cells depends on the proteolytic activity of Yta12 and the ATPase but not the proteolytic activity of Yta10ΔTM. Cells were grown at 30°C on YP medium containing glycerol. (A) Expression of a proteolytic site mutant variant of Yta10ΔTM in Δ_yta10_ cells (yta10 E559Q_Δ_TM) and in Δ_yta10_Δ_yta12_ cells expressing proteolytically inactive Yta12E614Q (yta10 E559Q_Δ_TM yta12 E614Q) (B) Expression of Yta10ΔTM variants harbouring mutations in conserved arginine residues of the SRH region in Δ_yta10_ cells.
Figure 3
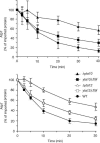
Impaired proteolysis of misfolded integral inner membrane proteins in yta10_Δ_TM and yta12_Δ_TM mitochondria. The stability of newly imported, 35S-labelled Yme2ΔC (A) and Oxa1ts (B) was analysed at 37°C in mitochondria isolated from wild-type (WT), Δ_yta10_, yta10_Δ_TM, Δ_yta12_ and yta12_Δ_TM cells. The average of at least three independent experiments (±s.e.m.) is shown.
Figure 4
Processing of Ccp1 by a mutant m_-AAA protease lacking the transmembrane domain of one subunit. Mitochondria were isolated from wild-type (WT), Δ_yta10, yta10_Δ_TM, Δ_yta12_ and yta12_Δ_TM cells and fractionated by SDS–PAGE (30 μg). Samples were analysed by immunoblotting using polyclonal antisera directed against Ccp1 (anti-Ccp1) and, as gel loading controls, cytochrome _b_2 (anti-Cyt b2) and Mge1 (anti-Mge1). p, precursor form of Ccp1; m, mature form of Ccp1.
Figure 5
Degradation of Atp7 by a mutant m_-AAA protease harbouring truncated subunits. Radiolabelled Atp7 was imported into mitochondria isolated from wild-type (WT), Δ_yta10, yta10_Δ_TM, Δ_yta12_ and yta12_Δ_TM cells, and the stability of newly imported Atp7 at 37°C was determined as in Fig 3. The average of four independent experiments (±s.e.m.) is shown.
Similar articles
- Electron cryomicroscopy structure of a membrane-anchored mitochondrial AAA protease.
Lee S, Augustin S, Tatsuta T, Gerdes F, Langer T, Tsai FT. Lee S, et al. J Biol Chem. 2011 Feb 11;286(6):4404-11. doi: 10.1074/jbc.M110.158741. Epub 2010 Dec 8. J Biol Chem. 2011. PMID: 21147776 Free PMC article. - Molecular insights into the _m_-AAA protease-mediated dislocation of transmembrane helices in the mitochondrial inner membrane.
Lee S, Lee H, Yoo S, Kim H. Lee S, et al. J Biol Chem. 2017 Dec 8;292(49):20058-20066. doi: 10.1074/jbc.M117.796763. Epub 2017 Oct 13. J Biol Chem. 2017. PMID: 29030426 Free PMC article. - m-AAA protease-driven membrane dislocation allows intramembrane cleavage by rhomboid in mitochondria.
Tatsuta T, Augustin S, Nolden M, Friedrichs B, Langer T. Tatsuta T, et al. EMBO J. 2007 Jan 24;26(2):325-35. doi: 10.1038/sj.emboj.7601514. EMBO J. 2007. PMID: 17245427 Free PMC article. - ATP-dependent proteases controlling mitochondrial function in the yeast Saccharomyces cerevisiae.
Van Dyck L, Langer T. Van Dyck L, et al. Cell Mol Life Sci. 1999 Nov 30;56(9-10):825-42. doi: 10.1007/s000180050029. Cell Mol Life Sci. 1999. PMID: 11212342 Free PMC article. Review. - m-AAA proteases, mitochondrial calcium homeostasis and neurodegeneration.
Patron M, Sprenger HG, Langer T. Patron M, et al. Cell Res. 2018 Mar;28(3):296-306. doi: 10.1038/cr.2018.17. Epub 2018 Feb 16. Cell Res. 2018. PMID: 29451229 Free PMC article. Review.
Cited by
- Identification of novel oxidized protein substrates and physiological partners of the mitochondrial ATP-dependent Lon-like protease Pim1.
Bayot A, Gareil M, Rogowska-Wrzesinska A, Roepstorff P, Friguet B, Bulteau AL. Bayot A, et al. J Biol Chem. 2010 Apr 9;285(15):11445-57. doi: 10.1074/jbc.M109.065425. Epub 2010 Feb 11. J Biol Chem. 2010. PMID: 20150421 Free PMC article. - Enhanced mitochondrial degradation of yeast cytochrome c with amphipathic structures.
Chen X, Moerschell RP, Pearce DA, Ramanan DD, Sherman F. Chen X, et al. Curr Genet. 2005 Feb;47(2):67-83. doi: 10.1007/s00294-004-0552-2. Epub 2004 Dec 17. Curr Genet. 2005. PMID: 15605252 - Mitochondrial protein quality control in health and disease.
Baker MJ, Palmer CS, Stojanovski D. Baker MJ, et al. Br J Pharmacol. 2014 Apr;171(8):1870-89. doi: 10.1111/bph.12430. Br J Pharmacol. 2014. PMID: 24117041 Free PMC article. Review. - Variable and tissue-specific subunit composition of mitochondrial m-AAA protease complexes linked to hereditary spastic paraplegia.
Koppen M, Metodiev MD, Casari G, Rugarli EI, Langer T. Koppen M, et al. Mol Cell Biol. 2007 Jan;27(2):758-67. doi: 10.1128/MCB.01470-06. Epub 2006 Nov 13. Mol Cell Biol. 2007. PMID: 17101804 Free PMC article. - NMR structure and MD simulations of the AAA protease intermembrane space domain indicates peripheral membrane localization within the hexaoligomer.
Ramelot TA, Yang Y, Sahu ID, Lee HW, Xiao R, Lorigan GA, Montelione GT, Kennedy MA. Ramelot TA, et al. FEBS Lett. 2013 Nov 1;587(21):3522-8. doi: 10.1016/j.febslet.2013.09.009. Epub 2013 Sep 18. FEBS Lett. 2013. PMID: 24055473 Free PMC article.
References
- Arlt H, Tauer R, Feldmann H, Neupert W, Langer T (1996) The YTA10–12-complex, an AAA protease with chaperone-like activity in the inner membrane of mitochondria. Cell 85: 875–885 - PubMed
- Bauer M, Behrens M, Esser K, Miachelis G, Pratje E (1994) PET1402, a nuclear gene required for proteolytic processing of cytochrome oxidase subunit 2 in yeast. Mol Gen Genet 245: 272–278 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases