JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis - PubMed (original) (raw)
. 2004 Jul;16(7):1938-50.
doi: 10.1105/tpc.022319. Epub 2004 Jun 18.
Affiliations
- PMID: 15208388
- PMCID: PMC514172
- DOI: 10.1105/tpc.022319
JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis
Oscar Lorenzo et al. Plant Cell. 2004 Jul.
Abstract
In spite of the importance of jasmonates (JAs) as plant growth and stress regulators, the molecular components of their signaling pathway remain largely unknown. By means of a genetic screen that exploits the cross talk between ethylene (ET) and JAs, we describe the identification of several new loci involved in JA signaling and the characterization and positional cloning of one of them, JASMONATE-INSENSITIVE1 (JAI1/JIN1). JIN1 encodes AtMYC2, a nuclear-localized basic helix-loop-helix-leucine zipper transcription factor, whose expression is rapidly upregulated by JA, in a CORONATINE INSENSITIVE1-dependent manner. Gain-of-function experiments confirmed the relevance of AtMYC2 in the activation of JA signaling. AtMYC2 differentially regulates the expression of two groups of JA-induced genes. The first group includes genes involved in defense responses against pathogens and is repressed by AtMYC2. Consistently, jin1 mutants show increased resistance to necrotrophic pathogens. The second group, integrated by genes involved in JA-mediated systemic responses to wounding, is activated by AtMYC2. Conversely, Ethylene-Response-Factor1 (ERF1) positively regulates the expression of the first group of genes and represses the second. These results highlight the existence of two branches in the JA signaling pathway, antagonistically regulated by AtMYC2 and ERF1, that are coincident with the alternative responses activated by JA and ET to two different sets of stresses, namely pathogen attack and wounding.
Figures
Figure 1.
Identification and Characterization of jai Mutants. (A) Sensitivity to JA of the ET-insensitive ein3-3 mutant, wild-type (Col-0), coi1-1, and alleles of all five complementation groups (jai1/jin1 to jai5/coi1) grown for 10 d on agar plates supplemented with 50 μM JA. (B) Chromosomal position of the five identified loci (jai1/jin1, jai2/jar1, jai3, jai4, and jai5/coi1). The position of jar1 and coi1 is also shown. cM, centimorgan. (C) Phenotypic comparison of the JA sensitivity of jin1-2 in wild-type and ein3-3 backgrounds. Plants were grown for 7 d on plates containing 50 μM JA. (D) Sensitivity of jin1 alleles (in wild-type background) to ABA. Seeds were germinated on plates containing 1 μM ABA, and the ABA-insensitive abi1-1 mutant and wild-type plants were included for comparison.
Figure 2.
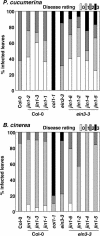
Susceptibility of jin1 Alleles to Necrotrophic Fungi. Graphical representation of disease symptoms 10 d after inoculation of the leaves with 2 × 106 spores/mL of P. cucumerina (top graph) or 5 d after inoculation of the leaves with 105 spores/mL of B. cinerea (bottom graph). Four-week-old plants of jin1 alleles in wild-type (Col-0) or ein3-3 backgrounds were used as well as the indicated mutants and wild-type plants. Disease rating was determined at the indicated days as described in Methods (0, resistance; 3, highly susceptible). Data values represent one of three independent experiments with similar results.
Figure 3.
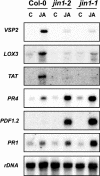
RNA Gel Blot Analysis of the Expression of JA-Regulated Genes in jin1 Alleles Compared with Wild-Type Plants. Fourteen-day-old wild-type plants and jin1-1 and jin1-2 mutants were not treated (C) or treated with 50 μM JA during 6 h. Ten micrograms of total RNA were loaded per lane, and the blot was hybridized with the indicated probes and rDNA as loading control.
Figure 4.
Molecular Identification of JIN1 by Positional Cloning. (A) Schematic representation of point mutations and T-DNA insertions in the jin1 alleles. Domains in the protein are indicated. aa, amino acids; M, Met; Z, stop codon. (B) RNA gel blot analysis of the defect in the level of JA-induced expression of AtMYC2 in jin1 alleles. All alleles in the top blot are in wild-type background, whereas the alleles in the bottom blot are in the ein3-3 background. Fourteen-day-old wild-type Arabidopsis seedlings were treated with water (C), 50 μM of JA, or nontreated (0) for 15 min. Twenty micrograms of total RNA were loaded per lane, and blots were hybridized with AtMYC2 probe and rDNA as loading control. (C) JA-insensitive phenotype of T-DNA insertion lines compared with wild-type (Col-0) and jin1-2 mutant grown on agar plates containing 50 μM JA.
Figure 5.
Constitutive Expression of AtMYC2. (A) Root growth inhibition and anthocyanin accumulation in two independent transgenic lines in each background (Col-0;35S:AtMYC2, jin1-1;35S:AtMYC2, and jin1-2;35S:AtMYC2) compared with parental plants (wild-type, jin1-1, and jin1-2) in the presence of different concentrations of JA (5 and 10 μM). (B) RNA gel blot analysis of the expression of AtMYC2 in the transgenic lines. Total RNA was extracted from 10-d-old wild-type plants, jin1-1 and jin1-2 mutants, and two independent transgenic lines in each genetic background (Col-0;35S:AtMYC2, jin1-1;35S:AtMYC2, and jin1-2;35S:AtMYC2). Ten micrograms of total RNA were loaded per lane, and the blot was hybridized with the AtMYC2 probe and rDNA as loading control. (C) Effect of ABA (0.3 μM) in seed germination and seedling development of 6-d-old wild-type Arabidopsis plants and two independent T2 transgenic lines (1 and 2) in each genetic background. C, control plants (no ABA treatment).
Figure 6.
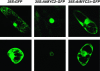
Subcellular Localization of AtMYC2 in Transiently Transformed BY2 Tobacco Cells. Constructs delivered correspond to C-terminal GFP fusions of full-length AtMYC2 (AtMYC2-GFP) and a truncated version corresponding to the mutation in the jin1-2 allele (AtMYC2Δ-GFP). The three top panels correspond to a longitudinal view of the cells, whereas the bottom panels represent transversal views. Nuclear and cytosolic distribution of the GFP protein alone is shown as control.
Figure 7.
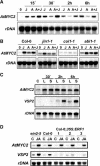
Stress and Hormonal Regulation of AtMYC2 Expression. (A) RNA gel blot analysis of the induction of AtMYC2 expression by JA and ABA. Ten-day-old wild-type Arabidopsis seedlings were treated with either 50 μM JA (J), 100 μM ABA (A), both (A+J), or nontreated (0) and tissue collected at the indicated times. Twenty micrograms of total RNA were loaded per lane, and blots were hybridized with AtMYC2 probe and rDNA as loading control. (B) RNA gel blot analysis of AtMYC2 induction by JA and ABA in different mutant backgrounds. Four-week-old wild-type plants and coi1, jin1-1, and abi1-1 mutants were treated with either 50 μM of jasmonic acid (J), 100 μM of ABA (A), both (A+J), or nontreated (0) and tissue collected after 30 min of treatment. Twenty micrograms of total RNA were loaded per lane, and blots were hybridized with the AtMYC2 probe and rDNA as loading control. (C) RNA gel blot analysis of AtMYC2 and VSP2 induction by wounding. Three-week-old wild-type plants were wounded, and samples from control leaves (C), local (L) damaged leaves, and systemic (S) nondamaged leaves were collected at the indicated times after wounding. (D) RNA gel blot analysis of the induction of AtMYC2 and VSP2 expression in 14-d-old wild-type, ein2-5, and three independent transgenic lines constitutively expressing ERF1, treated with JA (50 μM) for 30 min.
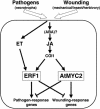
Figure 8.
Schematic Representation of the AtMYC2- and ERF1-Dependent Activation of Arabidopsis Responses to Pathogens and Wounding. Different types of stresses, such as wounding (mechanical or biotic) or necrotrophic pathogen infection, induce the synthesis and subsequent activation of the ET and JA pathways. JA alone will induce the expression of AtMYC2 that is responsible for the activation of wound-response genes and for the repression of pathogen-response genes. However, the cooperation of the ET and JA signals through the transcriptional induction of ERF1 drives to the activation of pathogen-response genes and to the repression of wounding-response genes (Lorenzo et al., 2003; this article). Therefore, the interplay between ERF1 and AtMYC2 allows the plant selection of the correct set of genes in response to these two stresses.
Similar articles
- Arabidopsis thaliana class-II TGA transcription factors are essential activators of jasmonic acid/ethylene-induced defense responses.
Zander M, La Camera S, Lamotte O, Métraux JP, Gatz C. Zander M, et al. Plant J. 2010 Jan;61(2):200-10. doi: 10.1111/j.1365-313X.2009.04044.x. Epub 2009 Oct 12. Plant J. 2010. PMID: 19832945 - The mitogen-activated protein kinase cascade MKK3-MPK6 is an important part of the jasmonate signal transduction pathway in Arabidopsis.
Takahashi F, Yoshida R, Ichimura K, Mizoguchi T, Seo S, Yonezawa M, Maruyama K, Yamaguchi-Shinozaki K, Shinozaki K. Takahashi F, et al. Plant Cell. 2007 Mar;19(3):805-18. doi: 10.1105/tpc.106.046581. Epub 2007 Mar 16. Plant Cell. 2007. PMID: 17369371 Free PMC article. - Conserved MYC transcription factors play a key role in jasmonate signaling both in tomato and Arabidopsis.
Boter M, Ruíz-Rivero O, Abdeen A, Prat S. Boter M, et al. Genes Dev. 2004 Jul 1;18(13):1577-91. doi: 10.1101/gad.297704. Genes Dev. 2004. PMID: 15231736 Free PMC article. - Regulation of gene expression by jasmonate hormones.
Memelink J. Memelink J. Phytochemistry. 2009 Sep;70(13-14):1560-70. doi: 10.1016/j.phytochem.2009.09.004. Epub 2009 Sep 30. Phytochemistry. 2009. PMID: 19796781 Review. - JAZ repressors set the rhythm in jasmonate signaling.
Chico JM, Chini A, Fonseca S, Solano R. Chico JM, et al. Curr Opin Plant Biol. 2008 Oct;11(5):486-94. doi: 10.1016/j.pbi.2008.06.003. Epub 2008 Jul 22. Curr Opin Plant Biol. 2008. PMID: 18653378 Review.
Cited by
- Identification of a novel jasmonate-responsive element in the AtJMT promoter and its binding protein for AtJMT repression.
Seo JS, Koo YJ, Jung C, Yeu SY, Song JT, Kim JK, Choi Y, Lee JS, Do Choi Y. Seo JS, et al. PLoS One. 2013;8(2):e55482. doi: 10.1371/journal.pone.0055482. Epub 2013 Feb 5. PLoS One. 2013. PMID: 23393583 Free PMC article. Retracted. - Tomato transcriptome and mutant analyses suggest a role for plant stress hormones in the interaction between fruit and Botrytis cinerea.
Blanco-Ulate B, Vincenti E, Powell AL, Cantu D. Blanco-Ulate B, et al. Front Plant Sci. 2013 May 14;4:142. doi: 10.3389/fpls.2013.00142. eCollection 2013. Front Plant Sci. 2013. PMID: 23717322 Free PMC article. - RGLG3 and RGLG4, novel ubiquitin ligases modulating jasmonate signaling.
Zhang X, Wu Q, An C. Zhang X, et al. Plant Signal Behav. 2012 Dec;7(12):1709-11. doi: 10.4161/psb.22144. Epub 2012 Oct 16. Plant Signal Behav. 2012. PMID: 23073017 Free PMC article. - BIN2 negatively regulates plant defence against Verticillium dahliae in Arabidopsis and cotton.
Song Y, Zhai Y, Li L, Yang Z, Ge X, Yang Z, Zhang C, Li F, Ren M. Song Y, et al. Plant Biotechnol J. 2021 Oct;19(10):2097-2112. doi: 10.1111/pbi.13640. Epub 2021 Jun 11. Plant Biotechnol J. 2021. PMID: 34036698 Free PMC article. - Post-translational modifications: emerging regulators manipulating jasmonate biosynthesis and signaling.
Yi R, Shan X. Yi R, et al. Plant Cell Rep. 2023 Feb;42(2):215-222. doi: 10.1007/s00299-022-02948-w. Epub 2022 Nov 27. Plant Cell Rep. 2023. PMID: 36436084 Review.
References
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301, 653–657. - PubMed
- Bell, C.J., and Ecker, J.R. (1994). Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics 19, 137–144. - PubMed
- Berger, S. (2002). Jasmonate-related mutants of Arabidopsis as tools for studying stress signaling. Planta 214, 497–504. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases