The roles of two IkappaB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection - PubMed (original) (raw)
The roles of two IkappaB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection
Hiroaki Hemmi et al. J Exp Med. 2004.
Abstract
Viral infection and stimulation with lipopolysaccharide (LPS) or double stranded RNA (dsRNA) induce phosphorylation of interferon (IFN) regulatory factor (IRF)-3 and its translocation to the nucleus, thereby leading to the IFN-beta gene induction. Recently, two IkappaB kinase (IKK)-related kinases, inducible IkappaB kinase (IKK-i) and TANK-binding kinase 1 (TBK1), were suggested to act as IRF-3 kinases and be involved in IFN-beta production in Toll-like receptor (TLR) signaling and viral infection. In this work, we investigated the physiological roles of these kinases by gene targeting. TBK1-deficient embryonic fibroblasts (EFs) showed dramatic decrease in induction of IFN-beta and IFN-inducible genes in response to LPS or dsRNA as well as after viral infection. However, dsRNA-induced expression of these genes was residually detected in TBK1-deficient cells and intact in IKK-i-deficient cells, but completely abolished in IKK-i/TBK1 doubly deficient cells. IRF-3 activation, in response not only to dsRNA but also to viral infection, was impaired in TBK1-deficient cells. Together, these results demonstrate that TBK1 as well as, albeit to a lesser extent, IKK-i play a crucial role in the induction of IFN-beta and IFN-inducible genes in both TLR-stimulated and virus-infected EFs.
Figures
Figure 1.
Generation of IKK-i −/− and TBK1−/− mice. (A) The structure of the IKK-i gene, the targeting vector, and the predicted mutated allele are shown. Closed boxes denote the coding exon. S, SphI. (B) Southern blot analysis of offspring from the heterozygote intercrosses. Genomic DNA was extracted from mouse tails, digested with SphI, electrophoresed, and hybridized with the radio-labeled probe indicated in A. Southern blotting gave a single 19-kbp band for wild type (+/+), a 4-kbp band for homozygous mutants (−/−), and both bands for heterozygous mice (+/−). (C) Northern blot analysis of thioglycollate-elicited peritoneal cells. Thioglycollate-elicited peritoneal cells from wild type and IKK-i −/− mice were stimulated with 100 ng/ml LPS for the indicated periods. Total RNA (10 μg) was electrophoresed, transferred to a nylon membrane, and hybridized using the IKK-i NH2-terminal fragment, the deleted region of IKK-i gene in targeting construct, TBK1, or β-actin cDNA fragment as a probe. (D) IFN-β promoter reporter assay. HEK293 cells were transiently cotransfected with the indicated expression vectors and the IFN-β luciferase reporter vector (0.1 μg). 36 h after transfection, luciferase activity in whole cell lysates was measured. (E) The structure of the TBK1 gene, the targeting vector, and the predicted mutated allele are shown. Closed boxes denote the coding exon. B, BamHI. (F) Southern blot analysis of EFs from the embryos of the heterozygous intercrosses. Genomic DNA was extracted from EFs, digested with BamHI, electrophoresed, and hybridized with the radiolabeled probe indicated in E. Southern blotting gave a single 3.5-kbp band for wild-type (+/+), a 1.2-kbp band for homozygous mutants (−/−), and both bands for heterozygous mice (+/−). (G) Northern blot analysis of EFs. Total RNA was extracted from wild-type and TBK1−/− EFs after stimulation with 1.0 μg/ml LPS for the indicated periods, electrophoresed, transferred to a nylon membrane, and hybridized using the IKK-i, TBK1, or β-actin cDNA fragment as a probe.
Figure 2.
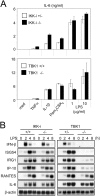
Impaired induction of IFN-β and IFN-inducible genes in LPS-stimulated TBK1−/−, but not IKK-i −/− EFs. (A) IL-6 production by EFs. Control (IKK-i +/− or TBK1+/+), IKK-i −/− (top), or TBK1−/− (bottom) EFs were stimulated with 10 ng/ml TNF-α, 10 ng/ml IL-1β, 100 ng/ml Pam3CSK4, or 1.0 or 10 μg/ml LPS for 24 h. The concentration of IL-6 in the culture supernatants was measured by ELISA. Data are shown as mean ± SD of triplicate samples of one representative experiment from three independent experiments. (B) Gene induction in LPS-stimulated EFs. Control (IKK-i +/+ or TBK1+/−), IKK-i −/− (left), or TBK1−/− (right) EFs were stimulated with 1.0 μg/ml LPS for the indicated periods. Total RNA was extracted and subjected to Northern blot analysis for the indicated genes. (C) Microarray analysis of LPS-stimulated EFs. Wild-type and TBK1−/− EFs were stimulated with 1.0 μg/ml LPS, and RNA was collected at the indicated time points and used to conduct microarray analysis. Absolute expression was displayed using Genespring software. The color code for absolute signal strength is indicated on the left.
Figure 2.
Impaired induction of IFN-β and IFN-inducible genes in LPS-stimulated TBK1−/−, but not IKK-i −/− EFs. (A) IL-6 production by EFs. Control (IKK-i +/− or TBK1+/+), IKK-i −/− (top), or TBK1−/− (bottom) EFs were stimulated with 10 ng/ml TNF-α, 10 ng/ml IL-1β, 100 ng/ml Pam3CSK4, or 1.0 or 10 μg/ml LPS for 24 h. The concentration of IL-6 in the culture supernatants was measured by ELISA. Data are shown as mean ± SD of triplicate samples of one representative experiment from three independent experiments. (B) Gene induction in LPS-stimulated EFs. Control (IKK-i +/+ or TBK1+/−), IKK-i −/− (left), or TBK1−/− (right) EFs were stimulated with 1.0 μg/ml LPS for the indicated periods. Total RNA was extracted and subjected to Northern blot analysis for the indicated genes. (C) Microarray analysis of LPS-stimulated EFs. Wild-type and TBK1−/− EFs were stimulated with 1.0 μg/ml LPS, and RNA was collected at the indicated time points and used to conduct microarray analysis. Absolute expression was displayed using Genespring software. The color code for absolute signal strength is indicated on the left.
Figure 3.
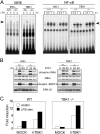
Requirement of TBK1 in LPS-induced ISRE-binding and activation of the IFN-β promoter. (A) Impaired ISRE-binding in TBK1−/− EFs. Control (IKK-i +/+ or TBK1+/−), IKK-i −/−, or TBK1−/− EFs were stimulated with 1.0 μg/ml LPS for the indicated periods, and ISRE binding (left) and NF-κB binding activity (right) were determined by EMSA. (B) Activation of MAP kinases in LPS-stimulated EFs. Control (IKK-i +/− or TBK1+/−), IKK-i −/−, or TBK1−/− EFs were stimulated with 10 μg/ml LPS for the times indicated. Whole cell lysates were prepared and blotted with antiphospho-JNK1/2 Ab (phospho-JNK1/2) or antiphospho-ERK1/2 Ab (phospho-ERK1/2). The total amounts of JNK1/2 and ERK1/2 were also determined. One representative experiment is shown. (C) The expression of TBK1 restored activation of IFN-β promoter in TBK1−/− EFs. TBK1+/+ (WT) or TBK1−/− EFs were cotransfected with human TBK1 (hTBK1) and the IFN-β promoter luciferase reporter. 24 h after transfection, the cells were stimulated with or without 10 μg/ml LPS for an additional 12 h before luciferase activity was measured. Similar results were obtained from two independent experiments.
Figure 4.
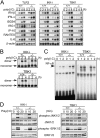
Impaired induction of IFN-β and IFN-inducible genes in poly(I:C)-stimulated TBK1−/−, but not IKK-i −/− EFs. (A) mRNA induction in poly(I:C)-stimulated EFs. Wild-type, TLR3−/−, IKK-i −/−, or TBK1−/− EFs were transfected with 10 μg/ml poly(I:C) for the indicated period. Total RNA was isolated and subjected to Northern blot analysis for the indicated genes. (B) Formation of IRF-3 dimer upon poly(I:C) stimulation. Control (IKK-i +/− or TBK1+/+), IKK-i −/−, or TBK1−/− EFs were transfected with 10 μg/ml poly(I:C) and incubated for the indicated periods. Whole cell extracts were prepared and subjected to native PAGE. Monomeric and dimeric IRF-3 were detected by Western blotting. (C) NF-κB DNA binding activity. Wild-type, IKK-i −/−, or TBK1−/− EFs were stimulated with 10 μg/ml poly(I:C) for the times indicated, and NF-κB binding was determined by EMSA. (D) MAP kinase activation in poly(I:C)-stimulated EFs. Control (IKK-i +/− or TBK1+/+), IKK-i −/−, or TBK1−/− EFs were stimulated with 10 μg/ml poly(I:C) for the indicated periods. Whole cell lysates were prepared and blotted with antiphospho-JNK1/2 (phospho-JNK1/2) or antiphospho-ERK1/2 Ab (phospho-ERK1/2). The total amounts of JNK1/2 and ERK1/2 were also determined. One representative experiment from two independent experiments is shown.
Figure 5.
Requirement of TBK1 for IFN-inducible gene expression in virus-infected EFs. (A) Control (IKK-i +/− or TBK1+/−), IKK-i −/−, or TBK1−/− EFs were infected with recombinant VSV or SeV for the indicated periods. Total RNA was extracted and subjected to Northern blot analysis with probes for the indicated genes. Similar results were obtained from three independent experiments. (B) Nuclear translocation of IRF-3 and NF-κB p65 in response to VSV infection. EFs were infected with recombinant VSV for the indicated periods, and nuclear proteins were extracted, separated by SDS-PAGE, and blotted with anti-IRF-3 and NF-κB p65 Abs.
Figure 6.
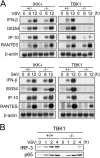
Involvement of IKK-i in IFN-β gene expression in poly(I:C) signaling. (A) Impaired mRNA induction in poly(I:C)-stimulated IKK-i −/−TBK1−/− cells. Immortalized control and IKK-i −/−TBK1−/− EFs were transfected with 10 μg/ml poly(I:C) and incubated for the indicated period. Total RNA was isolated and subjected to Northern blot analysis for the indicated genes. The similar results were obtained from two immortalized EFs. (B) Formation of IRF-3 dimer. Immortalized control (IKK-i +/−TBK1+/−) and IKK-i −/−TBK1−/− EFs were transfected with 10 μg/ml poly(I:C) and incubated for the indicated periods. Whole cell extracts were prepared and subjected to native PAGE. Monomeric and dimeric IRF-3 proteins were detected by Western blotting. The similar results were obtained from two independently established immortalized EFs. (C) NF-κB DNA binding activity. Immortalized IKK-i +/−TBK1+/− and IKK-i −/−TBK1−/− EFs were transfected with 10 μg/ml poly(I:C) for the indicated periods and NF-κB binding was determined by EMSA. Similar results were obtained from two lines of immortalized EFs. *, nonspecific bands. (D) MAP kinase activation. Immortalized IKK-i +/−TBK1+/− and IKK-i −/−TBK1−/− EFs were stimulated with 10 μg/ml poly(I:C) for the times indicated. Whole cell lysates were prepared and blotted with antiphospho-JNK1/2 Ab (phospho-JNK1/2) or antiphospho-ERK1/2 Ab (phospho-ERK1/2). The total amounts of JNK1/2 and ERK1/2 were also determined. One representative experiment from two independent experiments is shown.
Similar articles
- Differential requirement for TANK-binding kinase-1 in type I interferon responses to toll-like receptor activation and viral infection.
Perry AK, Chow EK, Goodnough JB, Yeh WC, Cheng G. Perry AK, et al. J Exp Med. 2004 Jun 21;199(12):1651-8. doi: 10.1084/jem.20040528. J Exp Med. 2004. PMID: 15210743 Free PMC article. - IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts.
McWhirter SM, Fitzgerald KA, Rosains J, Rowe DC, Golenbock DT, Maniatis T. McWhirter SM, et al. Proc Natl Acad Sci U S A. 2004 Jan 6;101(1):233-8. doi: 10.1073/pnas.2237236100. Epub 2003 Dec 16. Proc Natl Acad Sci U S A. 2004. PMID: 14679297 Free PMC article. - Interferon response induced by Toll-like receptor signaling.
Takeuchi O, Hemmi H, Akira S. Takeuchi O, et al. J Endotoxin Res. 2004;10(4):252-6. doi: 10.1179/096805104225005896. J Endotoxin Res. 2004. PMID: 15373970 Review. - Activation of TBK1 and IKKvarepsilon kinases by vesicular stomatitis virus infection and the role of viral ribonucleoprotein in the development of interferon antiviral immunity.
tenOever BR, Sharma S, Zou W, Sun Q, Grandvaux N, Julkunen I, Hemmi H, Yamamoto M, Akira S, Yeh WC, Lin R, Hiscott J. tenOever BR, et al. J Virol. 2004 Oct;78(19):10636-49. doi: 10.1128/JVI.78.19.10636-10649.2004. J Virol. 2004. PMID: 15367631 Free PMC article. - Regulation and function of IKK and IKK-related kinases.
Häcker H, Karin M. Häcker H, et al. Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
Cited by
- NS3 of bluetongue virus interferes with the induction of type I interferon.
Chauveau E, Doceul V, Lara E, Breard E, Sailleau C, Vidalain PO, Meurs EF, Dabo S, Schwartz-Cornil I, Zientara S, Vitour D. Chauveau E, et al. J Virol. 2013 Jul;87(14):8241-6. doi: 10.1128/JVI.00678-13. Epub 2013 May 8. J Virol. 2013. PMID: 23658442 Free PMC article. - Nonhematopoietic Nrf2 dominantly impedes adult progression of sickle cell anemia in mice.
Ghosh S, Ihunnah CA, Hazra R, Walker AL, Hansen JM, Archer DR, Owusu-Ansah AT, Ofori-Acquah SF. Ghosh S, et al. JCI Insight. 2016;1(4):e81090. doi: 10.1172/jci.insight.81090. Epub 2016 Apr 7. JCI Insight. 2016. PMID: 27158670 Free PMC article. - DNA methylation profiling identifies TBKBP1 as potent amplifier of cytotoxic activity in CMV-specific human CD8+ T cells.
Yu Z, Sasidharan-Nair V, Buchta T, Bonifacius A, Khan F, Pietzsch B, Ahmadi H, Beckstette M, Niemz J, Hilgendorf P, Mausberg P, Keller A, Falk C, Busch DH, Schober K, Cicin-Sain L, Müller F, Brinkmann MM, Eiz-Vesper B, Floess S, Huehn J. Yu Z, et al. PLoS Pathog. 2024 Sep 26;20(9):e1012581. doi: 10.1371/journal.ppat.1012581. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39325839 Free PMC article. - GADD34 suppresses lipopolysaccharide-induced sepsis and tissue injury through the regulation of macrophage activation.
Ito S, Tanaka Y, Oshino R, Okado S, Hori M, Isobe KI. Ito S, et al. Cell Death Dis. 2016 May 12;7(5):e2219. doi: 10.1038/cddis.2016.116. Cell Death Dis. 2016. PMID: 27171261 Free PMC article. - The Molecular Mechanism of Herpes Simplex Virus 1 UL31 in Antagonizing the Activity of IFN-β.
Gong L, Ou X, Hu L, Zhong J, Li J, Deng S, Li B, Pan L, Wang L, Hong X, Luo W, Zeng Q, Zan J, Peng T, Cai M, Li M. Gong L, et al. Microbiol Spectr. 2022 Feb 23;10(1):e0188321. doi: 10.1128/spectrum.01883-21. Epub 2022 Feb 23. Microbiol Spectr. 2022. PMID: 35196784 Free PMC article.
References
- Janeway, C.A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197–216. First published on October 4, 2001; 10.1146/annurev.immunol. 20.083001.084359. - PubMed
- Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335–376. First published on December 19, 2002; 10.1146/annurev.immunol.21.120601. 141126. - PubMed
- Takeuchi, O., S. Sato, T. Horiuchi, K. Hoshino, K. Takeda, Z. Dong, R.L. Modlin, and S. Akira. 2002. Role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J. Immunol. 169:10–14. - PubMed
- Takeuchi, O., T. Kawai, P.F. Muhlradt, M. Morr, J.D. Radolf, A. Zychlinsky, K. Takeda, and S. Akira. 2001. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 13:933–940. - PubMed
- Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 11:443–451. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous