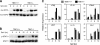Differential requirement for TANK-binding kinase-1 in type I interferon responses to toll-like receptor activation and viral infection - PubMed (original) (raw)
Differential requirement for TANK-binding kinase-1 in type I interferon responses to toll-like receptor activation and viral infection
Andrea K Perry et al. J Exp Med. 2004.
Abstract
TANK-binding kinase-1 (TBK1) and the inducible IkappaB kinase (IKK-i) have been shown recently to activate interferon (IFN) regulatory factor-3 (IRF3), the primary transcription factor regulating induction of type I IFNs. Here, we have compared the role and specificity of TBK1 in the type I IFN response to lipopolysaccharide (LPS), polyI:C, and viral challenge by examining IRF3 nuclear translocation, signal transducer and activator of transcription 1 phosphorylation, and induction of IFN-regulated genes. The LPS and polyI:C-induced IFN responses were abolished and delayed, respectively, in macrophages from mice with a targeted disruption of the TBK1 gene. When challenged with Sendai virus, the IFN response was normal in TBK1(-/-) macrophages, but defective in TBK1(-/-) embryonic fibroblasts. Although both TBK1 and IKK-i are expressed in macrophages, only TBK1 but not IKK-i was detected in embryonic fibroblasts by Northern blotting analysis. Furthermore, the IFN response in TBK1(-/-) embryonic fibroblasts can be restored by reconstitution with wild-type IKK-i but not a mutant IKK-i lacking kinase activity. Thus, our studies suggest that TBK1 plays an important role in the Toll-like receptor-mediated IFN response and is redundant with IKK-i in the response of certain cell types to viral infection.
Figures
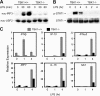
Figure 1.
TBK1 is required for LPS-mediated activation of type I IFN responses. TBK1+/+TNFR1+/− and TBK1−/−TNFR1−/− BMMs were stimulated with 10 ng/ml LPS for the indicated time points. (A) Nuclear fractions were probed for IRF3 and USF2 as a loading control for nuclear proteins. (B) Total cell extracts were probed for phospho-STAT1 and total STAT1. (C) Total RNA was extracted and analyzed by Q-PCR for expression of IFNβ, IP-10, IFNα5, IRF7, IL-15, and Mx1.
Figure 2.
TBK1-deficient BMMs have defective type I IFN responses to polyI:C. TBK1+/+TNFR1+/− and TBK1−/−TNFR1−/− BMMs were stimulated with 1 μg/ml polyI:C for the indicated time points. (A) Nuclear fractions were probed for IRF3 and USF2 as a loading control. (B) Total cell extracts were probed for phospho-STAT1 and total STAT1. (C) Total RNA was extracted and analyzed by Q-PCR for expression of IFNβ, IP-10, IFNα5, IRF7, IL-15, and Mx1.
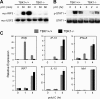
Figure 3.
TBK1-deficient BMMs induce normal NF-κB responses to LPS and polyI:C. TBK1+/+TNFR1+/− and TBK1−/−TNFR1−/− BMMs were stimulated with 10 ng/ml LPS or 1 μg/ml polyI:C for the indicated time points. (A) Nuclear fractions were probed for p65 and USF2 as a loading control. (B) EMSA was performed by incubating nuclear extracts were with an NF-κB-specific oligonucleotide. (C) Total RNA was extracted and analyzed by Q-PCR for expression of ICAM1 and IκBα.
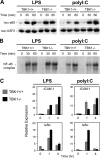
Figure 4.
TBK1-deficient BMMs have normal IFN responses to SeV. TBK1+/+TNFR1+/− and TBK1−/−TNFR1−/− BMMs were infected with SeV for the indicated time points. (A) Nuclear fractions were probed for IRF3 and USF2 as a loading control. (B) Total cell extracts were probed for phospho-STAT1 and total STAT1. (C) Total RNA was extracted and analyzed by Q-PCR for expression of IFNβ, IP-10, IFNα5, IRF7, IL-15, and Mx1.
Figure 5.
TBK1-deficient MEF cells have impaired IFN responses to SeV. Wild-type and TBK1−/− MEF cells were infected with SeV for the indicated time points. (A) Nuclear fractions were probed for IRF3 and USF2 as a loading control. Total cell extracts were probed for phospho-STAT1 and total STAT1. (B) Total RNA was extracted and analyzed by Q-PCR for expression of IFNβ, IP-10, IRF7, and IL-15. (C) EMSA was performed by incubating nuclear extracts were with an NF-κB-specific oligonucleotide. (D) Nuclear fractions were probed for p65 and USF2. (E) Total RNA was extracted and analyzed by Q-PCR for expression of ICAM1 and IκBα.
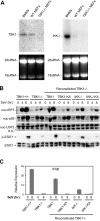
Figure 6.
IKK-i is differentially expressed in BMMs and MEFs, and can rescue a TBK1 deficiency during SeV infection. (A) Total RNA extracted from BMMs, wild-type MEFs, and TBK1−/− MEFs were probed by Northern blot for expression of TBK1 and IKK-i. 28 and 18 sRNA bands are shown as loading controls. (B) Wild-type MEFs, TBK1−/− MEFs, and TBK1−/− MEFs reconstituted with wild-type TBK1 or IKK-i, or their kinase-inactive (KA) mutants were infected with SeV for the time points indicated. Nuclear fractions were probed for IRF3, p65, and USF2 as a loading control. Total cell extracts were probed for phospho-STAT1 and total STAT1. (C) Cells were infected with SeV as in B and total RNA was extracted and analyzed by Q-PCR for expression of IFNβ.
Similar articles
- IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts.
McWhirter SM, Fitzgerald KA, Rosains J, Rowe DC, Golenbock DT, Maniatis T. McWhirter SM, et al. Proc Natl Acad Sci U S A. 2004 Jan 6;101(1):233-8. doi: 10.1073/pnas.2237236100. Epub 2003 Dec 16. Proc Natl Acad Sci U S A. 2004. PMID: 14679297 Free PMC article. - The roles of two IkappaB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection.
Hemmi H, Takeuchi O, Sato S, Yamamoto M, Kaisho T, Sanjo H, Kawai T, Hoshino K, Takeda K, Akira S. Hemmi H, et al. J Exp Med. 2004 Jun 21;199(12):1641-50. doi: 10.1084/jem.20040520. J Exp Med. 2004. PMID: 15210742 Free PMC article. - Activation of TBK1 and IKKvarepsilon kinases by vesicular stomatitis virus infection and the role of viral ribonucleoprotein in the development of interferon antiviral immunity.
tenOever BR, Sharma S, Zou W, Sun Q, Grandvaux N, Julkunen I, Hemmi H, Yamamoto M, Akira S, Yeh WC, Lin R, Hiscott J. tenOever BR, et al. J Virol. 2004 Oct;78(19):10636-49. doi: 10.1128/JVI.78.19.10636-10649.2004. J Virol. 2004. PMID: 15367631 Free PMC article. - Interferon response induced by Toll-like receptor signaling.
Takeuchi O, Hemmi H, Akira S. Takeuchi O, et al. J Endotoxin Res. 2004;10(4):252-6. doi: 10.1179/096805104225005896. J Endotoxin Res. 2004. PMID: 15373970 Review. - Regulation and function of IKK and IKK-related kinases.
Häcker H, Karin M. Häcker H, et al. Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
Cited by
- GADD34 suppresses lipopolysaccharide-induced sepsis and tissue injury through the regulation of macrophage activation.
Ito S, Tanaka Y, Oshino R, Okado S, Hori M, Isobe KI. Ito S, et al. Cell Death Dis. 2016 May 12;7(5):e2219. doi: 10.1038/cddis.2016.116. Cell Death Dis. 2016. PMID: 27171261 Free PMC article. - TCR signaling to NF-κB and mTORC1: Expanding roles of the CARMA1 complex.
Shi JH, Sun SC. Shi JH, et al. Mol Immunol. 2015 Dec;68(2 Pt C):546-57. doi: 10.1016/j.molimm.2015.07.024. Epub 2015 Aug 8. Mol Immunol. 2015. PMID: 26260210 Free PMC article. Review. - The role of immunostimulatory nucleic acids in septic shock.
Bleiblo F, Michael P, Brabant D, Ramana CV, Tai T, Saleh M, Parrillo JE, Kumar A, Kumar A. Bleiblo F, et al. Int J Clin Exp Med. 2012;5(1):1-23. Epub 2012 Jan 15. Int J Clin Exp Med. 2012. PMID: 22328944 Free PMC article. - Toll-like receptor 2-dependent endosomal signaling by Staphylococcus aureus in monocytes induces type I interferon and promotes intracellular survival.
Musilova J, Mulcahy ME, Kuijk MM, McLoughlin RM, Bowie AG. Musilova J, et al. J Biol Chem. 2019 Nov 8;294(45):17031-17042. doi: 10.1074/jbc.RA119.009302. Epub 2019 Sep 26. J Biol Chem. 2019. PMID: 31558608 Free PMC article. - Interferons direct an effective innate response to Legionella pneumophila infection.
Plumlee CR, Lee C, Beg AA, Decker T, Shuman HA, Schindler C. Plumlee CR, et al. J Biol Chem. 2009 Oct 30;284(44):30058-66. doi: 10.1074/jbc.M109.018283. Epub 2009 Aug 31. J Biol Chem. 2009. PMID: 19720834 Free PMC article.
References
- Muller, U., U. Steinhoff, L.F. Reis, S. Hemmi, J. Pavlovic, R.M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science. 264:1918–1921. - PubMed
- Stark, G.R., I.M. Kerr, B.R. Williams, R.H. Silverman, and R.D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227–264. - PubMed
- Taniguchi, T., K. Ogasawara, A. Takaoka, and N. Tanaka. 2001. IRF family of transcription factors as regulators of host defense. Annu. Rev. Immunol. 19:623–655. - PubMed
- Taniguchi, T., and A. Takaoka. 2002. The interferon-alpha/beta system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors. Curr. Opin. Immunol. 14:111–116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM08042/GM/NIGMS NIH HHS/United States
- T32 GM008042/GM/NIGMS NIH HHS/United States
- R37 AI047868/AI/NIAID NIH HHS/United States
- R01 CA087924/CA/NCI NIH HHS/United States
- R37 AI47868/AI/NIAID NIH HHS/United States
- R01 AI056154/AI/NIAID NIH HHS/United States
- R01 CA87924/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous