Cargo- and compartment-selective endocytic scaffold proteins - PubMed (original) (raw)
Review
Cargo- and compartment-selective endocytic scaffold proteins
Iwona Szymkiewicz et al. Biochem J. 2004.
Abstract
The endocytosis of membrane receptors is a complex and tightly controlled process that is essential for maintaining cellular homoeostasis. The removal of receptors from the cell surface can be constitutive or ligand-induced, and occurs in a clathrin-dependent or -independent manner. The recruitment of receptors into specialized membrane domains, the formation of vesicles and the trafficking of receptors together with their ligands within endocytic compartments are regulated by reversible protein modifications, and multiple protein-protein and protein-lipid interactions. Recent reports describe a variety of multidomain molecules that facilitate receptor endocytosis and function as platforms for the assembly of protein complexes. These scaffold proteins typically act in a cargo-specific manner, recognizing one or more receptor types, or function at the level of endocytic cellular microcompartments by controlling the movement of cargo molecules and linking endocytic machineries to signalling pathways. In the present review we summarize present knowledge on endocytic scaffold molecules and discuss their functions.
Figures
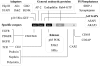
Figure 1. CIN85 is an endocytic scaffold
CIN85 is an example of a multimeric adaptor that is involved in cargo internalizaton and sorting. Through its multiple domains, CIN85 associates with a number of proteins, which are divided here into functional groups. CIN85 oligomerizes and simultaneously binds various molecules, changing its conformation and binding partners upon cell stimulation, thus co-ordinating the trafficking of several cargo proteins. Abbreviations: STAP, stem-cell-specific adaptor protein containing pleckstrin homology and SH2 domains; BLNK, B cell linker protein; SHIP-1, SH2-containing inositol phosphatase-1; GEF, guanine nucleotide exchange factor; ASAP1, Arf-GTPase-activating protein 1; ARAP3, Arf GAP and Rho GAP with ankyrin repeat and pleckstrin homology domains protein 3; PDGFR, platelet-derived growth factor receptor; HGFR, hepatocyte growth factor receptor; PI3K, phosphoinositide 3-kinase; PAK, p21-activated kinase; SFK, Src family kinase; CC, coiled-coil.
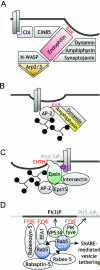
Figure 2. Examples of dynamic protein–lipid complexes that couple the cargo with the biogenesis and trafficking of vesicles in endocytic microcompartments
(A) Endophilin co-ordinates cargo entry and actin polymerization at clathrin-coated pits. Its BAR domain senses and induces membrane curvature. (B) The BAR domain of amphiphysin binds to curved negatively charged phospholipids, while its other domains associate with AP-2, clathrin and dynamin. (C) Epsin interacts with clathrin, AP-2, Eps15, intersectin and ubiquitin (purple oval). Its ENTH domain binds PtdIns(4,5)P_2 [PI(4,5)P2] and introduces membrane curvature. (D) The Rab domain at the level of the early endosome is a functional protein multimer that is coupled to the membrane phospholipid PtdIns3_P via binding of FYVE-domain-containing proteins. For details, see the text.
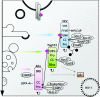
Figure 3. Ubiquitin traffic
Lysosomal degradation of ubiquitinated receptors is directed by the sequential action of the ubiquitin-binding protein complex composed of Hrs, Tsg101 and Alix with associated proteins. All three molecules form oligomers. Hrs and Tsg101 bind ubiquitin directly via UIM (ubiquitin-interacting motif) and UEV (ubiquitin-conjugating enzyme E2 variant) domains respectively. Hrs binds PtdIns3_P_ [PI(3)P] and sequesters cargo in clathrin-coated regions of early endosomes, preventing it from recycling. The UEV domain of Tsg101 associates with PSAP motifs in Hrs and Alix (block arrows). Tsg101 and Alix co-ordinate the function of ESCRT protein complexes involved in the biogenesis of MVBs. Alix associates with LPBA, a lipid that is necessary for the formation of inner vesicles of MVBs. Upon ligand stimulation, several accessory proteins, including Hrs, undergo mono-ubiquitnation (indicated by a purple asterisk). This has been implicated in co-ordinating vesicular transport. The endocytic sorting machinery is recruited during the budding of RNA viruses, whose Gag proteins are ubiquitinated and have motifs that bind Tsg101 and Alix. Abbreviations: VHS, Vsp27p, Hrs, STAM; CC, coiled-coil; SNAP-25, 25 kDa synaptosome-associated protein; BRD, Bro1-rhophilin conserved domain; CHMP4, charged MVB protein; ALG, apoptosis-linked gene 2.
Similar articles
- Specialised endocytic proteins regulate diverse internalisation mechanisms and signalling outputs in physiology and cancer.
Giangreco G, Malabarba MG, Sigismund S. Giangreco G, et al. Biol Cell. 2021 Apr;113(4):165-182. doi: 10.1111/boc.202000129. Epub 2020 Dec 30. Biol Cell. 2021. PMID: 33617023 Review. - The interplay between clathrin-coated vesicles and cell signalling.
Mills IG. Mills IG. Semin Cell Dev Biol. 2007 Aug;18(4):459-70. doi: 10.1016/j.semcdb.2007.07.001. Epub 2007 Jul 6. Semin Cell Dev Biol. 2007. PMID: 17692542 Review. - Epsins: adaptors in endocytosis?
Wendland B. Wendland B. Nat Rev Mol Cell Biol. 2002 Dec;3(12):971-7. doi: 10.1038/nrm970. Nat Rev Mol Cell Biol. 2002. PMID: 12461563 Review. - Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis.
Shih SC, Katzmann DJ, Schnell JD, Sutanto M, Emr SD, Hicke L. Shih SC, et al. Nat Cell Biol. 2002 May;4(5):389-93. doi: 10.1038/ncb790. Nat Cell Biol. 2002. PMID: 11988742 - Regulation of Src trafficking and activation by the endocytic regulatory proteins MICAL-L1 and EHD1.
Reinecke JB, Katafiasz D, Naslavsky N, Caplan S. Reinecke JB, et al. J Cell Sci. 2014 Apr 15;127(Pt 8):1684-98. doi: 10.1242/jcs.133892. Epub 2014 Jan 30. J Cell Sci. 2014. PMID: 24481818 Free PMC article.
Cited by
- Endoplasmic reticulum-localized human papillomavirus type 16 E5 protein alters endosomal pH but not trans-Golgi pH.
Disbrow GL, Hanover JA, Schlegel R. Disbrow GL, et al. J Virol. 2005 May;79(9):5839-46. doi: 10.1128/JVI.79.9.5839-5846.2005. J Virol. 2005. PMID: 15827198 Free PMC article. - Mediation of clathrin-dependent trafficking during cytokinesis and cell expansion by Arabidopsis stomatal cytokinesis defective proteins.
McMichael CM, Reynolds GD, Koch LM, Wang C, Jiang N, Nadeau J, Sack FD, Gelderman MB, Pan J, Bednarek SY. McMichael CM, et al. Plant Cell. 2013 Oct;25(10):3910-25. doi: 10.1105/tpc.113.115162. Epub 2013 Oct 31. Plant Cell. 2013. PMID: 24179130 Free PMC article. - Structural diversity and differential expression of novel human intersectin 1 isoforms.
Kropyvko S, Gerasymchuk D, Skrypkina I, Dergai M, Dergai O, Nikolaienko O, Rynditch A, Tsyba L. Kropyvko S, et al. Mol Biol Rep. 2010 Jul;37(6):2789-96. doi: 10.1007/s11033-009-9824-8. Epub 2009 Sep 24. Mol Biol Rep. 2010. PMID: 19777371 - NPFXD-mediated endocytosis is required for polarity and function of a yeast cell wall stress sensor.
Piao HL, Machado IM, Payne GS. Piao HL, et al. Mol Biol Cell. 2007 Jan;18(1):57-65. doi: 10.1091/mbc.e06-08-0721. Epub 2006 Oct 25. Mol Biol Cell. 2007. PMID: 17065552 Free PMC article. - Interaction between Epsin/Yap180 adaptors and the scaffolds Ede1/Pan1 is required for endocytosis.
Maldonado-Báez L, Dores MR, Perkins EM, Drivas TG, Hicke L, Wendland B. Maldonado-Báez L, et al. Mol Biol Cell. 2008 Jul;19(7):2936-48. doi: 10.1091/mbc.e07-10-1019. Epub 2008 Apr 30. Mol Biol Cell. 2008. PMID: 18448668 Free PMC article.
References
- Herbst J. J., Opresko L. K., Walsh B. J., Lauffenburger D. A., Wiley H. S. Regulation of postendocytic trafficking of the epidermal growth factor receptor through endosomal retention. J. Biol. Chem. 1994;269:12865–12873. - PubMed
- Wiley H. S., Herbst J. J., Walsh B. J., Lauffenburger D. A., Rosenfeld M. G., Gill G. N. The role of tyrosine kinase activity in endocytosis, compartmentation, and down-regulation of the epidermal growth factor receptor. J. Biol. Chem. 1991;266:11083–11094. - PubMed
- Murthy V. N., De Camilli P. Cell biology of the presynaptic terminal. Annu. Rev. Neurosci. 2003;26:701–728. - PubMed
- Gundelfinger E. D., Kessels M. M., Qualmann B. Temporal and spatial coordination of exocytosis and endocytosis. Nat. Rev. Mol. Cell Biol. 2003;4:127–139. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources