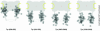Nucleoporins as components of the nuclear pore complex core structure and Tpr as the architectural element of the nuclear basket - PubMed (original) (raw)
Nucleoporins as components of the nuclear pore complex core structure and Tpr as the architectural element of the nuclear basket
Sandra Krull et al. Mol Biol Cell. 2004 Sep.
Abstract
The vertebrate nuclear pore complex (NPC) is a macromolecular assembly of protein subcomplexes forming a structure of eightfold radial symmetry. The NPC core consists of globular subunits sandwiched between two coaxial ring-like structures of which the ring facing the nuclear interior is capped by a fibrous structure called the nuclear basket. By postembedding immunoelectron microscopy, we have mapped the positions of several human NPC proteins relative to the NPC core and its associated basket, including Nup93, Nup96, Nup98, Nup107, Nup153, Nup205, and the coiled coil-dominated 267-kDa protein Tpr. To further assess their contributions to NPC and basket architecture, the genes encoding Nup93, Nup96, Nup107, and Nup205 were posttranscriptionally silenced by RNA interference (RNAi) in HeLa cells, complementing recent RNAi experiments on Nup153 and Tpr. We show that Nup96 and Nup107 are core elements of the NPC proper that are essential for NPC assembly and docking of Nup153 and Tpr to the NPC. Nup93 and Nup205 are other NPC core elements that are important for long-term maintenance of NPCs but initially dispensable for the anchoring of Nup153 and Tpr. Immunogold-labeling for Nup98 also results in preferential labeling of NPC core regions, whereas Nup153 is shown to bind via its amino-terminal domain to the nuclear coaxial ring linking the NPC core structures and Tpr. The position of Tpr in turn is shown to coincide with that of the nuclear basket, with different Tpr protein domains corresponding to distinct basket segments. We propose a model in which Tpr constitutes the central architectural element that forms the scaffold of the nuclear basket.
Figures
Figure 1.
Peptide antibody target sites on Nups and Tpr. Relative positions of target sites for peptide antibodies used in this study are indicated by arrowheads. Black boxes represent sequence segments for which probability of coiled-coil formation is predicted to be >50%. Blue boxes represent the C2-C2 zinc fingers that constitute the Ran-GTP binding domain of Nup153. Brown vertical stripes in the Nup153 and Nup98 diagrams indicate individual FxFG and GFxF sequence motifs, and red verticals represent GLFG motifs and derivatives thereof present only in Nup98. Simple FG repeats are not included. The Nup98 and Nup96 diagrams represent the products of Nup196 autoproteolysis. The shaded boxes in the Nup96 diagram represent the N-terminal binding site for Nup98, and the Sec13 binding region. The shaded boxes in the Nup98 diagram stand for the binding site for the mRNA transport factor Rae1/Gle2, and the C-terminal binding region for Nup96 and Nup88. The shaded box in the Nup153 diagram represents the overlapping bindings regions for the Nup160 subcomplex (aa 39–339), Tpr (aa 228–439), Nup50 (337–611), and the RNA binding domain (250–400). The green rectangle encloses the Tpr binding domain of Nup153 and the turquoise rectangle the NPC/Nup153 binding domain of Tpr.
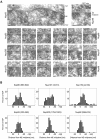
Figure 2.
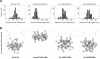
Postembedding immunogold-EM of nucleoporins in HeLa cells. (A) Representative examples of LR White-embedded NEs after labeling with peptide-specific antibodies against Nup93 aa 350–369, Nup96 aa 880–900, Nup98 aa 596–618, Nup107 aa 33–51, Nup153 aa 22–36, and Nup205 aa 1784–1803, and 10-nm gold-coupled secondary antibodies. Cells were fixed with FA plus GA. Cytoplasm (Cyt) and nuclear compartment (Nuc), separated by the NE, are oriented toward top and bottom, respectively. Arrows (top left) and brackets (right) demark some NPCs in cross section. Bars, 50 nm. Same magnification for top left image and image panels below. (B) Distribution of immunogold particles (IGP) relative to the NE midplane (dashed vertical). Data collection was from randomly chosen gold-labeled NEs from encoded specimens and values were normalized for NE width variations as described in Figure 3A. Histograms include all grains detected in proximity to perpendicularly sectioned NPCs up to 100 nm from the NE midplane at the cytoplasmic and 150 nm at the nuclear side (negative values). Additional specific labeling of distinct structures deep within the nuclear interior, such as centromere labeling with α-Nup107 (Belgareh et al., 2001) and labeling of GLFG bodies with α-Nup98 (Griffis et al., 2002) was not considered. Sample sizes (n) are 75 (Nup93), 78 (Nup96), 109 (Nup98), 105 (Nup107), 116 (Nup153), and 98 (Nup205). In sporadic cases in which two gold particles <25 nm apart labeled the same NPC, their mean distance value was included, treating them as an immunogold cluster that labeled only one site. Very rare clusters of three or more grains were not considered. Use of α-Nup153 aa 22–36 on GA-fixed specimen produced some additional staining throughout the cell that was rated as background also observed far beyond the borders of the histogram. When compared at a 1:1 surface ratio, the approximate level (dotted gray horizontal) of such background in the cytoplasmic and nuclear compartments reached ∼8–11% of the peak distribution at the nuclear side of the NE.
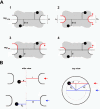
Figure 3.
Normalization of gold grain coordinates for NE width variations and nondiametric perpendicular section planes through NPCs. (A) Variations in NE width may occur naturally or as a result of the fixation and dehydration process that may cause some shrinkage of the NE's perinuclear lumen. Local swelling and increase in NE width can be seen as well, although less frequently. The dimensions of the NPC proper are less subjected to such alterations. However, because the NE midplane is used as the _x_-axis relative to which the distance of an individual gold grain is measured, variations in NE width can cause deviations between the relative position of a grain in the picture and when incorporated according to its measured distance d into an idealistic NPC model (A1). In principle, swelling or shrinkage (red arrows) of the NE might affect both inner and outer NE membrane positions similarly (A2). In this case, the midplane would still be the same as the original mid-axis (mporig) in the idealistic model A1. However, the inner NE membrane and its position relative to the NPC are more likely to be stabilized by interactions with the underlying lamina and heterochromatin. In this scenario, it is mainly the relative position of the outer NE membrane that will be affected by shrinkage or swelling of the perinuclear lumen (A3, A4). This alters the position of the midline (mpalt) between both membranes. To take such deviations into account, the width of the NE, including perinuclear lumen and membranes, was measured on each side of a gold-grain decorated NPC. This yielded the two values h1 and h2. The width H for the idealistic NE was set at 32 nm. The normalized distance value D for each gold grain located on the nuclear side of the midplane was then calculated by using the equation D = d + [(H – h1) + (H – h2)] 2–2; for grains on the cytoplasmic side the equation was D = d + [(H – h1) + (H – h2)] 2–2 (B) Perpendicular section planes through most gold-labeled NPCs do not pass directly through the NPC center but represent chords that are shorter than the real NPC diameter. Consequently, the measured mean distance between gold grains and apparent vertical mid-axis (_y_-axis) through cross-sectioned NPCs is smaller than the real radial distance of the target protein from the NPC's eightfold rotational symmetry axis. This deviation can be accounted for by measuring not only the distance between apparent mid-axis and gold grain but also the apparent diameter of this NPC in cross section. The real distance Dy between gold-labeled target and real mid-axis can then be calculated using the equation , respectively:
. R stands for the real NPC radius, i.e., the distance between mid axis and membrane boundary of an ideal NPC (here set at 40 nm), r for the measured distance between apparent mid-axis and membrane boundary of the cross-sectioned NPC, and d for the measured distance between apparent mid-axis and gold grain. Grains on grazing sections of the pore where r was <25 nm were not considered.
Figure 4.
Relative distributions of immunogold label for different nucleoporins with respect to an idealistic NPC in cross section. Data collection was from at least 50 randomly chosen gold-labeled NPCs in which both membrane boundaries flanking the pore channel were discernible. The coordinates for each immunogold particle (IGP) were normalized for NE width variations and nondiametric perpendicular section planes as described in Figure 3. Because of the eightfold rotational symmetry of the NPC, assignment of x values to either side of the _y_-axis is arbitrary. Therefore, the algebraic signs of a few x values were reversed to allocate similar numbers of grains to each side of the _y_-axis. To further enhance clarity of peak distributions and reduce signal spread due to extremes of antibody spreading or minor background label, sporadic grains most distant from the calculated mean position for each Nup (Table 1) were not included (cut-off ≤5%). Red dots mark the NPC center, flanked by the NPC core structures, including the coaxial rings, and the NE membrane (yellow). Attached to the nuclear ring, only two of the eight rod-like fibers of the nuclear basket, and the distal terminal ring (dashed circle) are depicted. Pore diameter in this scheme corresponds to 80 nm, NE width to 32 nm, and distance between NE midplane and terminal ring to 80 nm.
Figure 5.
Postembedding immunogold localization of Tpr domains relative to the NPC in HeLa cells. (A) Representative examples of LR White-embedded NEs after labeling with peptidespecific antibodies against Tpr aa 636–655, 914–932, 2063–2084, and 2338–2363. The nuclear compartment is oriented toward the bottom. Arrows and arrowheads demark some NPCs in cross and grazing section, respectively. Bar, 50 nm, for all micrographs. Cells were fixed with FA plus GA. (B) Distribution of gold particles relative to the NE midplane. Data were from randomly chosen gold-labeled NEs from encoded specimens. Histograms include all grains detected in proximity to perpendicularly sectioned NPCs up to 50 nm from the NE midplane at the cytoplasmic and 200 nm at the nuclear side. Values were normalized for NE width variations as described in Figure 3A. Sample sizes (n) are 101 (636–655), 92 (914–932), 172 (2063–2084), and 121 (2338–2363). The histogram for α-Tpr 2063–2084 combines two highly similar data sets from separate labelings with antibodies from one animal. For α-Tpr 636–655, only one of two similar data sets are shown, obtained with different batches of Tpr 636–655 antibodies from two animals (see Figure 7).
Figure 6.
Distributions of gold grains relative to NPCs after immunogold labeling of different Tpr domains. Details of schemes and presentation of gold grains collected from cells fixed with FA plus GA are as for Figure 4. The coordinates for each IGP were normalized for NE width variations and nondiametric perpendicular section planes through NPCs as described in Figure 3. To enhance clarity of peak distributions, sporadic gold particles most distant from the normalized mean position for each Tpr segment (Table 2) were not included (cut-off ≤5%).
Figure 7.
Characterization of novel peptide-specific Tpr antibodies. (A) Immunoblotting of HeLa cell proteins by using antibodies against Tpr aa 636–655 and aa 914–932. Proteins were separated by SDS-PAGE and stained with Ponceau S (PS) after transfer to nitrocellulose filters. Lanes include total cell proteins (TP), soluble proteins first extracted with digitonin (D) and then with Triton X-100 (T), and residual cell sediment proteins (S). Lanes D, T, and S were loaded with the same percentage of each fraction. Immunodetection of Tpr by enhanced chemiluminescence reaction was on filters identical or similar to the ones stained with Ponceau S. Positions of marker proteins are given on the left margin. Note that both antibodies label one major band of >250 kDa (large arrow), in line with Tpr's mass of 267 kDa. Only weak cross-reactions of α-Tpr 914–932 with a detergent-insoluble protein of ∼135 kDa, and of α-Tpr 636–655 with detergent-soluble cytoplasmic proteins of ∼32 and ∼22 kDa (asterisks) are noted after prolonged exposure. These latter proteins were not recognized by a second batch of Tpr 636–655 antibodies raised in another guinea pig, which revealed other minor cross-reactions instead (unpublished data). (B) Confocal IFM of HeLa cells immunolabeled with α-Tpr 636–655 and 914–932. Cells were permeabilized with detergent either after or before fixation. DNA-staining with TO-PRO-3 is in blue. Bar, 20 μm.
Figure 8.
Postembedding immunogold localization of Tpr domains and the Tpr-binding domain of Nup153 in FA-fixed HeLa cells. (A) Nonnormalized distributions of gold grains relative to the NE midplane, after immunogold-labeling of FA-fixed HeLa cells with antibodies against Tpr aa 8–21, 1622–1640, and 2338–2363, and against Nup153 aa 391–404. Data were collected from randomly chosen gold-labeled NEs from encoded specimens. Histograms include all gold particles detected in proximity to perpendicularly sectioned NPCs, up to 50 nm from the NE midplane at the cytoplasmic and 200 nm at the nuclear side. Because membrane boundaries and perinuclear lumen of the NE in FA-fixed specimen are often not clearly discriminable, values were not normalized for NE width variations. Sample sizes (n) are 55 (Nup153), 58 (Tpr 8–21), 63 (Tpr 1622–1640), and 62 (Tpr 2338–2363). (B) Nonnormalized distributions of gold grains relative to nuclear pores. Membrane borders of the pore are generally difficult to pinpoint in FA-fixed specimens, which usually prevents accurate measurements of pore diameters. IGP positions were therefore superimposed onto the scheme of a representative pore in cross section as described previously (Walther et al., 2002), without normalization for nondiametric perpendicular section planes and NE width variations. To enhance clarity of peak distributions, gold grains most distant from the approximate nonnormalized mean positions (Table 3) were not included (cut-off ≤5% for α-Nup153, α-Tpr 1622–1640, and α-Tpr 2338–2363; 9% for α-Tpr 8–21). Immunogold labelings were performed with several GA-insensitive Nup and Tpr antibodies in parallel, to allow double blind comparative experiments with ample encryption of specimen.
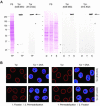
Figure 9.
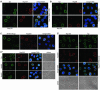
Temporally distinct loss of different NPC components after RNAi of Nup96, Nup107, and Nup205. HeLa cells were studied by confocal IFM at day 3.5 posttransfection with Nup107 (A) and Nup 96 (B) siRNAs, and at day 2 posttransfection with Nup205 siRNAs (C–C″), by using α-Nup96 aa 880–900, α-Nup107 aa 33–51, α-Nup93 aa 2–218, α-Nup205 aa 1784–1803, α-Tpr 2063–2084 (A, C′–C″), α-Nup 153 aa 22–36 (B), Nup153 mAb PF190 × 7A8 (A and C′), Tpr mAb 203–37 (B), and mAb 414 against Nup62 and other FxFG-repeat nucleoporins. Cells were permeabilized with Triton X-100 after (A and B) or before fixation (C–C″). (A and B) Cells transfected with siRNAs for Nup96 or 107 show no or only traces of the target protein at day 3.5 posttransfection; at this point, staining for Tpr and Nup153 is strongly reduced as well. Bright NE staining for these proteins is visible only in few cells that have remained untransfected. In contrast to Tpr- and Nup153-deficient nuclei that still permit different types of nucleocytoplasmic transport as well as nuclear growth during interphase (Hase and Cordes, 2003; our unpublished data), the majority of Nup96- and 107-deficient nuclei are characterized by small size indicative of nuclear growth arrest. (C) Cells transfected with siRNAs for Nup205 are already largely devoid of the target protein at day 2 posttransfection and already then often exhibit small-sized nuclei. At this point, NE staining intensity with mAb 414 in most transfected cells is also strongly diminished, whereas (C′) staining for Tpr and Nup153 is often reduced only moderately. Notably, NEs of cells in early G1 phase (some marked by brackets) can be largely depleted of Nup205 and nevertheless be clearly positive for Nup153 and Tpr, indicative that their binding to the NPC does not require stoichiometric amounts of Nup205. Two nontransfected Nup205-positive pairs of cells in early G1 (asterisks) are shown as reference. (C″) Nup205 deficiency impairs Nup93 incorporation into newly assembled NEs in early telophase (Hase and Cordes, 2003), resulting in strongly reduced Nup93 staining in postmitotic NEs (bracket). A nontransfected pair of cells in early G1 positive for both Nup205 and Nup93 (asterisk), and a Nup205-positive pair of telophase cells (arrowhead) in which late reincoporation of Tpr into the NE (Hase and Cordes, 2003) has not yet occurred are shown as reference. Bars, 20 μm; same magnification in A, B, and in C′, C″.
Figure 10.
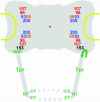
Model for nucleoporins as NPC core components and Tpr as the architectural element of the nuclear basket. Approximate mean positions of immunogold-labeled protein domains of Nups and Tpr, deduced from Figures 4 and 6, are superimposed onto the scheme of an idealistic NPC. The relative position of the Nup98 target domain, close to the Nup93 subcomplex, is shown slightly offset compared with its mean coordinates, due to spatial limitations. All Nups but 153 are regarded as bilateral symmetrically arranged on both sides of the NE midplane, without excluding that some Nups might locate in more than two planes, or that others like Nup205 might locate only in one plane closer to or equivalent to the midplane. The present degree of resolution also does not allow clarifying whether Nups located in the cytoplasmic plane are pointing into the same or opposite directions as their counterparts on the nuclear side. Furthermore, the actual distances from the eightfold rotational symmetry axis might be slightly shorter for some Nups and Tpr domains, and larger for others (Supplemental Figure S7). Relative positions of the flexible FG repeat domains of Nup98 and 153 were not a subject of this study and might locate in areas distant from the Nup98 and 153 domains targeted in this work. In this model, the basket fibers are composed of coiled-coiled Tpr homodimers or oligomers that fold back on themselves, thereby forming tetrameric or oligomeric intramolecular assemblies, with the first and second half of the rod domains arranged in an antiparallel manner. The region where Tpr bends backwards (arrow) is located close to its NBD that tethers the protein to the nuclear coaxial ring, mediated by interaction with the N-terminal domain of Nup153. Distal segments of the Tpr rod domains and the C-terminal tail domains are proposed to represent the main constituents of the terminal ring. Relative positions of Tpr N terminus (NT) and C terminus (CT) are indicated.
Similar articles
- Direct interaction with nup153 mediates binding of Tpr to the periphery of the nuclear pore complex.
Hase ME, Cordes VC. Hase ME, et al. Mol Biol Cell. 2003 May;14(5):1923-40. doi: 10.1091/mbc.e02-09-0620. Mol Biol Cell. 2003. PMID: 12802065 Free PMC article. - Structural characterization of altered nucleoporin Nup153 expression in human cells by thin-section electron microscopy.
Duheron V, Chatel G, Sauder U, Oliveri V, Fahrenkrog B. Duheron V, et al. Nucleus. 2014;5(6):601-12. doi: 10.4161/19491034.2014.990853. Nucleus. 2014. PMID: 25485891 Free PMC article. - Novel vertebrate nucleoporins Nup133 and Nup160 play a role in mRNA export.
Vasu S, Shah S, Orjalo A, Park M, Fischer WH, Forbes DJ. Vasu S, et al. J Cell Biol. 2001 Oct 29;155(3):339-54. doi: 10.1083/jcb.200108007. Epub 2001 Oct 29. J Cell Biol. 2001. PMID: 11684705 Free PMC article. - Function and assembly of nuclear pore complex proteins.
Bodoor K, Shaikh S, Enarson P, Chowdhury S, Salina D, Raharjo WH, Burke B. Bodoor K, et al. Biochem Cell Biol. 1999;77(4):321-9. Biochem Cell Biol. 1999. PMID: 10546895 Review. - Structural dynamics of the nuclear pore complex.
Sakiyama Y, Panatala R, Lim RYH. Sakiyama Y, et al. Semin Cell Dev Biol. 2017 Aug;68:27-33. doi: 10.1016/j.semcdb.2017.05.021. Epub 2017 Jun 1. Semin Cell Dev Biol. 2017. PMID: 28579449 Review.
Cited by
- The molecular architecture of the nuclear basket.
Singh D, Soni N, Hutchings J, Echeverria I, Shaikh F, Duquette M, Suslov S, Li Z, van Eeuwen T, Molloy K, Shi Y, Wang J, Guo Q, Chait BT, Fernandez-Martinez J, Rout MP, Sali A, Villa E. Singh D, et al. Cell. 2024 Sep 19;187(19):5267-5281.e13. doi: 10.1016/j.cell.2024.07.020. Epub 2024 Aug 9. Cell. 2024. PMID: 39127037 Free PMC article. - A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells.
Roux KJ, Kim DI, Raida M, Burke B. Roux KJ, et al. J Cell Biol. 2012 Mar 19;196(6):801-10. doi: 10.1083/jcb.201112098. Epub 2012 Mar 12. J Cell Biol. 2012. PMID: 22412018 Free PMC article. - Asi1 is an inner nuclear membrane protein that restricts promoter access of two latent transcription factors.
Boban M, Zargari A, Andréasson C, Heessen S, Thyberg J, Ljungdahl PO. Boban M, et al. J Cell Biol. 2006 Jun 5;173(5):695-707. doi: 10.1083/jcb.200601011. Epub 2006 May 30. J Cell Biol. 2006. PMID: 16735580 Free PMC article. - Tpr Deficiency Disrupts Erythroid Maturation With Impaired Chromatin Condensation in Zebrafish Embryogenesis.
Wu S, Chen K, Xu T, Ma K, Gao L, Fu C, Zhang W, Jing C, Ren C, Deng M, Chen Y, Zhou Y, Pan W, Jia X. Wu S, et al. Front Cell Dev Biol. 2021 Oct 13;9:709923. doi: 10.3389/fcell.2021.709923. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34722501 Free PMC article. - Proxiome assembly of the plant nuclear pore reveals an essential hub for gene expression regulation.
Tang Y, Yang X, Huang A, Seong K, Ye M, Li M, Zhao Q, Krasileva K, Gu Y. Tang Y, et al. Nat Plants. 2024 Jun;10(6):1005-1017. doi: 10.1038/s41477-024-01698-9. Epub 2024 May 21. Nat Plants. 2024. PMID: 38773271
References
- Allen, T.D., Cronshaw, J.M., Bagley, S., Kiseleva, E., and Goldberg, M.W. (2000). The nuclear pore complex: mediator of translocation between nucleus and cytoplasm. J. Cell Sci. 113, 1651–1659. - PubMed
- Arlucea, J., Andrade, R., Alonso, R., and Arechaga, J. (1998). The nuclear basket of the nuclear pore complex is part of a higher-order filamentous network that is related to chromatin. J. Struct. Biol. 124, 51–58. - PubMed
- Bayliss, R., Littlewood, T., and Stewart, M. (2000). Structural basis for the interaction between FxFG nucleoporin repeats and importin-β in nuclear trafficking. Cell 102, 99–108. - PubMed
- Bendayan, M., Nanci, A., and Kan, F.W.K. (1987). Effect of tissue processing on colloidal gold cytochemistry. J. Histochem. Cytochem. 35, 983–996. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous