POT1-interacting protein PIP1: a telomere length regulator that recruits POT1 to the TIN2/TRF1 complex - PubMed (original) (raw)
Comparative Study
. 2004 Jul 15;18(14):1649-54.
doi: 10.1101/gad.1215404. Epub 2004 Jul 1.
Affiliations
- PMID: 15231715
- PMCID: PMC478187
- DOI: 10.1101/gad.1215404
Comparative Study
POT1-interacting protein PIP1: a telomere length regulator that recruits POT1 to the TIN2/TRF1 complex
Jeffrey Zheng-Sheng Ye et al. Genes Dev. 2004.
Abstract
Human telomere length is controlled by a negative feedback loop based on the binding of TRF1 to double-stranded telomeric DNA. The TRF1 complex recruits POT1, a single-stranded telomeric DNA-binding protein necessary for cis-inhibition of telomerase. By mass spectrometry, we have identified a new telomeric protein, which we have named POT1-interacting protein 1 (PIP1). PIP1 bound both POT1 and the TRF1-interacting factor TIN2 and could tether POT1 to the TRF1 complex. Reduction of PIP1 or POT1 levels with shRNAs led to telomere elongation, indicating that PIP1 contributes to telomere length control through recruitment of POT1.
Copyright 2004 Cold Spring Harbor Laboratory Press ISSN
Figures
Figure 1.
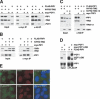
Interactions of PIP1 with TIN2 and POT1 and localization of PIP1 to telomeres. (A) Co-IP of PIP1 with POT1 and TIN2. Epitope-tagged constructs were transfected into 293T cells in various combinations (top) and subjected to coIP using antibodies to the myc or HA tags. Protein expression levels are shown in input lysates. Immunoblots were done with the myc antibody to detect POT1 and the Flag antibody to detect PIP1 and TIN2. (B) Co-IP of PIP1 with TIN2 but not TRF1. The indicated constructs were cotransfected into 293T cells, and the anti-myc (PIP1) IPs were analyzed for the presence of PIP1 (myc antibody), TRF1, and TIN2 (Flag antibody for both). (C) PIP1 does not interact with TIN2-13. N-FH2-TIN2 or N-FH2-TIN2-13 were coexpressed with Flag-PIP1 and myc-TRF1 as indicated. Proteins recovered in the anti-HA immunoprecipitate were analyzed by immunoblotting. TRF1 was detected with the myc antibody, and PIP1, TIN2, and TIN2-13 were detected with the Flag antibody. (D) PIP1 interacts with POT1ΔOB. Flag-PIP1 was coexpressed with myc-tagged full-length POT1 or POT1ΔOB. IPs were performed with Flag-antibody and analyzed by immunoblotting with antibodies to the epitope tags. (E) Telomeric localization of exogenous PIP1. IF of HeLa1.2.11 cells expressing Flag-PIP1 from a retrovirus (pLPC). PIP1 was detected with anti-Flag (M2; green). TRF1 (top) and TIN2 (bottom) were detected with Abs 371 and 865, respectively (red). DNA was stained with DAPI (shown in the merged panel).
Figure 2.
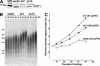
Telomere elongation induced by RNAi-mediated reduction of POT1. (A) Immunoblot of HTC75 cells expressing the indicated shRNAs (vector control represents cells infected with the empty virus) at PD50 postselection. POT1 was detected with Ab 978 as described previously (Loayza and de Lange 2003). The asterisk marks a cross-reacting band that serves as a loading control. (B) Telomeric restriction fragment blot of the cell lines shown in A performed at the indicated population doublings (PDs). The molecular mass in kilobases of HindIII-digested λ DNA fragments is shown on the left. (C) Graph of the mean telomeric restriction fragment length of the indicated cell lines plotted versus PD. Elongation rates of the telomeres are indicated to the right.
Figure 3.
Telomere elongation induced by RNAi-mediated reduction of PIP1. (A) HTC75 cells expressing myc-tagged PIP1 were transfected with siRNA targeting luciferase (control) and two independent siRNAs targeting PIP1 (#3 and #4). Myc-PIP1 levels were detected by immunoblotting using 9E10 (Oncogene) with α-tubulin as loading control. (B) Telomere lengthening caused by PIP1 knockdown. Telomeric restriction fragment blot of the HTC75 cells infected with PIP1 shRNA retroviruses or Flag-tagged PIP1. Population doublings (PDs) are indicated above the lanes. The molecular mass in kilobases of HindIII-digested λ DNA fragments is shown on the left. (C) Graph of the mean telomeric restriction fragment length of the indicated cell lines plotted versus PD. Elongation rates of the telomeres are indicated to the right.
Figure 4.
Summary of the TRF1-TIN2-PIP1-POT1 telomere length regulation pathway. (A) Components of the TRF1 complex and their interactions with DNA and/or proteins. The PIP1 interaction with TIN2 has not been mapped in detail but is abolished by deletion of the indicated domain. (B) Schematic of POT1-mediated telomere length regulation, emphasizing the protein interactions leading to recruitment of POT1. It is possible (likely) that some or all of the components of the TRF1 complex are associated with each other prior to the binding of TRF1 to telomeric DNA. It is not known whether POT1 can bind to single-stranded DNA while interacting with PIP1.
Similar articles
- TIN2 binds TRF1 and TRF2 simultaneously and stabilizes the TRF2 complex on telomeres.
Ye JZ, Donigian JR, van Overbeek M, Loayza D, Luo Y, Krutchinsky AN, Chait BT, de Lange T. Ye JZ, et al. J Biol Chem. 2004 Nov 5;279(45):47264-71. doi: 10.1074/jbc.M409047200. Epub 2004 Aug 16. J Biol Chem. 2004. PMID: 15316005 - Structure, dynamics, and regulation of TRF1-TIN2-mediated trans- and cis-interactions on telomeric DNA.
Pan H, Kaur P, Barnes R, Detwiler AC, Sanford SL, Liu M, Xu P, Mahn C, Tang Q, Hao P, Bhattaram D, You C, Gu X, Lu W, Piehler J, Xu G, Weninger K, Riehn R, Opresko PL, Wang H. Pan H, et al. J Biol Chem. 2021 Sep;297(3):101080. doi: 10.1016/j.jbc.2021.101080. Epub 2021 Aug 14. J Biol Chem. 2021. PMID: 34403696 Free PMC article. - POT1 as a terminal transducer of TRF1 telomere length control.
Loayza D, De Lange T. Loayza D, et al. Nature. 2003 Jun 26;423(6943):1013-8. doi: 10.1038/nature01688. Epub 2003 May 25. Nature. 2003. PMID: 12768206 - Telomere biology: a new player in the end zone.
Colgin L, Reddel R. Colgin L, et al. Curr Biol. 2004 Oct 26;14(20):R901-2. doi: 10.1016/j.cub.2004.09.075. Curr Biol. 2004. PMID: 15498484 Review. - Regulation of telomerase by telomeric proteins.
Smogorzewska A, de Lange T. Smogorzewska A, et al. Annu Rev Biochem. 2004;73:177-208. doi: 10.1146/annurev.biochem.73.071403.160049. Annu Rev Biochem. 2004. PMID: 15189140 Review.
Cited by
- Telomeres as hotspots for innate immunity and inflammation.
Nassour J, Przetocka S, Karlseder J. Nassour J, et al. DNA Repair (Amst). 2024 Jan;133:103591. doi: 10.1016/j.dnarep.2023.103591. Epub 2023 Nov 5. DNA Repair (Amst). 2024. PMID: 37951043 Free PMC article. Review. - Cockayne Syndrome group B protein interacts with TRF2 and regulates telomere length and stability.
Batenburg NL, Mitchell TR, Leach DM, Rainbow AJ, Zhu XD. Batenburg NL, et al. Nucleic Acids Res. 2012 Oct;40(19):9661-74. doi: 10.1093/nar/gks745. Epub 2012 Aug 16. Nucleic Acids Res. 2012. PMID: 22904069 Free PMC article. - HOT1 is a mammalian direct telomere repeat-binding protein contributing to telomerase recruitment.
Kappei D, Butter F, Benda C, Scheibe M, Draškovič I, Stevense M, Novo CL, Basquin C, Araki M, Araki K, Krastev DB, Kittler R, Jessberger R, Londoño-Vallejo JA, Mann M, Buchholz F. Kappei D, et al. EMBO J. 2013 Jun 12;32(12):1681-701. doi: 10.1038/emboj.2013.105. Epub 2013 May 17. EMBO J. 2013. PMID: 23685356 Free PMC article. - Targets of histone H3 lysine 9 methyltransferases.
Levinsky AJ, McEdwards G, Sethna N, Currie MA. Levinsky AJ, et al. Front Cell Dev Biol. 2022 Dec 6;10:1026406. doi: 10.3389/fcell.2022.1026406. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36568972 Free PMC article. Review. - POT1-TPP1 enhances telomerase processivity by slowing primer dissociation and aiding translocation.
Latrick CM, Cech TR. Latrick CM, et al. EMBO J. 2010 Mar 3;29(5):924-33. doi: 10.1038/emboj.2009.409. Epub 2010 Jan 21. EMBO J. 2010. PMID: 20094033 Free PMC article.
References
- Ancelin K., Brunori, M., Bauwens, S., Koering, C.E., Brun, C., Ricoul, M., Pommier, J.P., Sabatier, L., and Gilson, E. 2002. Targeting assay to study the cis functions of human telomeric proteins: Evidence for inhibition of telomerase by TRF1 and for activation of telomere degradation by TRF2. Mol. Cell. Biol. 22: 3474-3487. - PMC - PubMed
- Baumann P. and Cech, T.R. 2001. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 292: 1171-1175. - PubMed
- Chong L., van Steensel, B., Broccoli, D., Erdjument-Bromage, H., Hanish, J., Tempst, P., and de Lange, T. 1995. A human telomeric protein. Science 270: 1663-1667. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P41 RR000862/RR/NCRR NIH HHS/United States
- K08 CA093604/CA/NCI NIH HHS/United States
- K08 CA93604/CA/NCI NIH HHS/United States
- T32 CA009673/CA/NCI NIH HHS/United States
- RR00862/RR/NCRR NIH HHS/United States
- T32 CA09673-26A1/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials