Germ cell aneuploidy in zebrafish with mutations in the mitotic checkpoint gene mps1 - PubMed (original) (raw)
Germ cell aneuploidy in zebrafish with mutations in the mitotic checkpoint gene mps1
Kenneth D Poss et al. Genes Dev. 2004.
Abstract
Aneuploidy, resulting from chromosome missegregation during meiosis, is a major cause of human infertility and birth defects. However, its molecular basis remains incompletely understood. Here we have identified a spectrum of chromosome anomalies in embryos of zebrafish homozygous for a hypomorphic mutation in Mps1, a kinase required for the mitotic checkpoint. These aneuploidies are caused by meiotic error and result in severe developmental defects. Our results reveal Mps1 as a critical regulator of chromosome number in zebrafish, and demonstrate how slight genetic perturbation of a mitotic checkpoint factor can dramatically reduce the fidelity of chromosome segregation during vertebrate meiosis.
Figures
Figure 1.
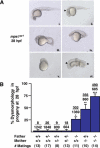
Zebrafish homozygous for mps1zp1 generate dysmorphic offspring. (A) One-quarter to one-third of embryos from mps1zp1 parents appeared normal at 28 hpf (panel i). A significant proportion of embryos displayed head degeneration (panel ii, arrowhead), whereas others displayed severe defects in head, somite, and trunk development (panels iii,iv). In a small percentage of embryos, no head or trunk was distinguishable (panel v), or spectacular patterning defects were noted (panel vi). This embryo has two joined head and trunk structures in the absence of posterior structures (arrow-heads point to otic vesicles). (B) Frequency of dysmorphic progeny from different combinations of wild-type (+/+), mps1zp1 heterozygous (+/-), and mps1zp1 homozygous (-/-) parents. Data are shown as mean ± S.E.M. Student's _t_-test, (*) p  0.001 versus two +/+ parents. (**) p
0.001 versus two +/+ parents. (**) p  0.001 versus two +/+ parents, p < 0.05 versus -/- father and +/+ mother. (***) p
0.001 versus two +/+ parents, p < 0.05 versus -/- father and +/+ mother. (***) p  0.001 versus two +/+ parents, p
0.001 versus two +/+ parents, p  0.001 versus -/- father and +/+ mother, p < 0.05 versus +/+ father and -/- mother. Numbers above the error bars represent the total number of dysmorphic embryos among the crosses, divided by the total number of embryos analyzed. The majority of mps1zp1 animals in these experiments were 7–10 mo of age. In our facilities, zebrafish retain fecundity from 4 to 15 mo of age, and live 2–3 yr.
0.001 versus -/- father and +/+ mother, p < 0.05 versus +/+ father and -/- mother. Numbers above the error bars represent the total number of dysmorphic embryos among the crosses, divided by the total number of embryos analyzed. The majority of mps1zp1 animals in these experiments were 7–10 mo of age. In our facilities, zebrafish retain fecundity from 4 to 15 mo of age, and live 2–3 yr.
Figure 2.
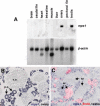
mps1 is localized to immature zebrafish germ cells. (A) Northern analysis of mps1 expression using several adult tissues. RNA samples from testis and ovary, although slightly overloaded on this blot, are the only tissues that demonstrate mps1 expression. (B) In situ hybridization of ovarian tissue section detects strong mps1 expression (violet stain) in small, immature perinucleolar oocytes (p.o.). Mature vitellogenic oocytes (v.o.) show little or no mps1 expression. (C) In testis, mps1 mRNA is localized to primary and secondary spermatocytes (sc), with little or no expression in spermatozoa (sz). To confirm the identity of _mps1_-positive cells, animals were injected with BrdU 5 h prior to tissue processing. In 5 h, BrdU-positive cells (pink) result from mitotic division of spermatogonial stem cells to yield primary spermatocytes, or mitotic division plus one meiotic division to yield secondary spermatocytes. All BrdU-positive cells were also _mps1_-positive; mature spermatozoa were BrdU- and _mps1_-negative.
Figure 3.
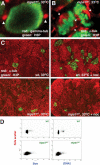
Mitotic checkpoint deficits and altered chromosome content in germ cells of male mps1zp1 zebrafish. (A) Confocal image of a meiotic cell from the testis of an mps1zp1 male treated for 12 h at 33°C, stained for γ-tubulin (red) and H3P (green). Arrowheads indicate two centrosomes. (B) Confocal image of two meiotic cells from the testis of an mps1zp1 male treated for 12 h at 33°C, stained for α-tubulin (red) and H3P (green). Each mitotic spindle has two clear poles (arrowheads). (C) Sections of testes from wild-type (top) and mps1zp1 (bottom) zebrafish treated with (left) or without (right) nocodazole for 12 h at 33°C, stained for α-tubulin (red) and H3P (green). Nocodazole treatment activates the mitotic checkpoint and induces arrest in wild-type animals, indicated by increases in meiotic cells, but has no effect in mps1zp1 clutchmates. (D) FACS analysis of sperm from representative wild-type (top) and mps1zp1 (bottom) males. mps1zp1 sperm displayed a wide range of size and DNA content (as assessed by propidium iodide fluorescence).
Figure 4.
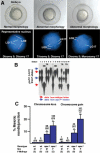
A high frequency of aneuploidy in mps1zp1 germ lines. (A) Eight-somite stage embryos (top), and representative FISH analyses (bottom) indicating one DAPI-stained nucleus for linkage groups 9 (red probe) and 17 (green). (Left) Wild-type embryo with elongated body, tapered tail, and normal head structure, is disomic for both linkage groups. (Center) mps1zp1 embryo with degenerated head and malformed trunk and tail, is trisomic for LG9. (Right) mps1zp1 embryo with blunted head and tail is monosomic for LG17. (B) Auto-radiograph indicating genotypes for genetic marker gof12 on LG17, from dysmorphic progeny of a representative wild-type male × mps1zp1 female cross. Each lane represents one embryo. The two parents of this family displayed four differently sized gof12 PCR products; red arrows indicate two alleles from the wild-type father, whereas blue arrows indicate two alleles from the mps1zp1 mother. Note two embryos with maternal LG17 monosomy (MO) and three with maternal LG17 trisomy (TR). (C) Meiotic nondisjunction rates of wild-type and mps1zp1 fathers and mothers in wild-type × mps1zp1 crosses. Data were compiled from analysis of 273 dysmorphic embryos from mps1zp1 fathers, and 197 dysmorphic embryos from mps1zp1 mothers. The numbers above the error bars represent the total number of losses (monosomy) or gains (trisomy) among five chromosomes analyzed, divided by the total number of chromosomes with informative polymorphisms. Student's _t_-test, (*) p  0.001 versus wild-type male or female. (**) p
0.001 versus wild-type male or female. (**) p  0.001 versus wild-type male or female, p < 0.05 versus mps1zp1 father. All parents used for FISH and genotyping analyses were maintained at 25°C.
0.001 versus wild-type male or female, p < 0.05 versus mps1zp1 father. All parents used for FISH and genotyping analyses were maintained at 25°C.
Similar articles
- Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint.
Abrieu A, Magnaghi-Jaulin L, Kahana JA, Peter M, Castro A, Vigneron S, Lorca T, Cleveland DW, Labbé JC. Abrieu A, et al. Cell. 2001 Jul 13;106(1):83-93. doi: 10.1016/s0092-8674(01)00410-x. Cell. 2001. PMID: 11461704 - Inhibition of Plk1 induces mitotic infidelity and embryonic growth defects in developing zebrafish embryos.
Jeong K, Jeong JY, Lee HO, Choi E, Lee H. Jeong K, et al. Dev Biol. 2010 Sep 1;345(1):34-48. doi: 10.1016/j.ydbio.2010.06.004. Epub 2010 Jun 8. Dev Biol. 2010. PMID: 20553902 - Depletion of Aurora-A in zebrafish causes growth retardation due to mitotic delay and p53-dependent cell death.
Jeon HY, Lee H. Jeon HY, et al. FEBS J. 2013 Mar;280(6):1518-30. doi: 10.1111/febs.12153. Epub 2013 Feb 24. FEBS J. 2013. PMID: 23351126 - Leader of the SAC: molecular mechanisms of Mps1/TTK regulation in mitosis.
Pachis ST, Kops GJPL. Pachis ST, et al. Open Biol. 2018 Aug;8(8):180109. doi: 10.1098/rsob.180109. Open Biol. 2018. PMID: 30111590 Free PMC article. Review. - Faulty spindle checkpoint and cohesion protein activities predispose oocytes to premature chromosome separation and aneuploidy.
Mailhes JB. Mailhes JB. Environ Mol Mutagen. 2008 Oct;49(8):642-58. doi: 10.1002/em.20412. Environ Mol Mutagen. 2008. PMID: 18626998 Review.
Cited by
- Cellular abundance of Mps1 and the role of its carboxyl terminal tail in substrate recruitment.
Sun T, Yang X, Wang W, Zhang X, Xu Q, Zhu S, Kuchta R, Chen G, Liu X. Sun T, et al. J Biol Chem. 2010 Dec 3;285(49):38730-9. doi: 10.1074/jbc.M110.177642. Epub 2010 Sep 30. J Biol Chem. 2010. PMID: 20884615 Free PMC article. - Degradation of the human mitotic checkpoint kinase Mps1 is cell cycle-regulated by APC-cCdc20 and APC-cCdh1 ubiquitin ligases.
Cui Y, Cheng X, Zhang C, Zhang Y, Li S, Wang C, Guadagno TM. Cui Y, et al. J Biol Chem. 2010 Oct 22;285(43):32988-32998. doi: 10.1074/jbc.M110.140905. Epub 2010 Aug 20. J Biol Chem. 2010. PMID: 20729194 Free PMC article. - Genetic regulation of sex determination and maintenance in zebrafish (Danio rerio).
Kossack ME, Draper BW. Kossack ME, et al. Curr Top Dev Biol. 2019;134:119-149. doi: 10.1016/bs.ctdb.2019.02.004. Epub 2019 Mar 21. Curr Top Dev Biol. 2019. PMID: 30999973 Free PMC article. Review. - Structural and mechanistic insights into Mps1 kinase activation.
Wang W, Yang Y, Gao Y, Xu Q, Wang F, Zhu S, Old W, Resing K, Ahn N, Lei M, Liu X. Wang W, et al. J Cell Mol Med. 2009 Aug;13(8B):1679-1694. doi: 10.1111/j.1582-4934.2008.00605.x. J Cell Mol Med. 2009. PMID: 19120698 Free PMC article. - The MPS1 family of protein kinases.
Liu X, Winey M. Liu X, et al. Annu Rev Biochem. 2012;81:561-85. doi: 10.1146/annurev-biochem-061611-090435. Epub 2012 Apr 5. Annu Rev Biochem. 2012. PMID: 22482908 Free PMC article. Review.
References
- Abrieu A., Magnaghi-Jaulin, L., Kahana, J.A., Peter, M., Castro, A., Vigneron, S., Lorca, T., Cleveland, D.W., and Labbe, J.C. 2001. Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint. Cell 106: 83-93. - PubMed
- Cleveland D.W., Mao, Y., and Sullivan, K.F. 2003. Centromeres and kinetochores: From epigenetics to mitotic checkpoint signaling. Cell 112: 407-421. - PubMed
- Dobles M., Liberal, V., Scott, M.L., Benezra, R., and Sorger, P.K. 2000. Chromosome missegregation and apoptosis in mice lacking the mitotic checkpoint protein Mad2. Cell 101: 635-645. - PubMed
- Fisk H.A. and Winey, M. 2001. The mouse Mps1p-like kinase regulates centrosome duplication. Cell 106: 95-104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases